An understanding of the embryologic origins of the retrorectal space is essential to the management of the congenital retrorectal tumors. The majority of these tumors arise from developmental abnormalities: persistence of embryologic remnants, defects in midline fusion, or embryologic sequestration [16].
The primitive gut develops from the endoderm of the yolk sac as a straight tube, ultimately differentiating into three separate regions – the foregut, midgut, and hindgut – by the fourth week of gestation [17–19]. The hindgut eventually extends from the distal one third of the transverse colon to the anal canal proximal to the dentate line. At the level of the pubococcygeal line, the hindgut unites with the ventrally located allantois to create the cloaca (“sewer”), a cul-de-sac [18]. In the early embryo, the hindgut continues beyond the cloaca, where it is known as the tailgut, or postanal gut, that is, the early embryo possesses a true tail that is best defined at 35 days of gestation (8 mm in length) (Fig. 50.2) [10, 20]. Between the fourth and sixth weeks of gestation, the urorectal septum descends to meet the cloacal membrane, the junction of the endoblast and the ectoblast, thus dividing the cloaca into the urogenital sinus anteriorly and the upper anorectal canal posteriorly [21]. The cloacal membrane, which is the future site of the anus, regresses via a process of programmed cell death, allowing for communication with the amniotic fluid [21, 22]. Similarly, the tailgut, located caudad to the future anus, normally completely disintegrates by 56 days of gestation (35 mm in length) [10, 20, 22]. The persistence of the postanal gut generates a tailgut cyst. Because the carcinoid tumors that arise in the retrorectal space are predominantly associated with tailgut cysts, Ghosh and colleagues [23] suggest that the two lesions may share a common hindgut origin.
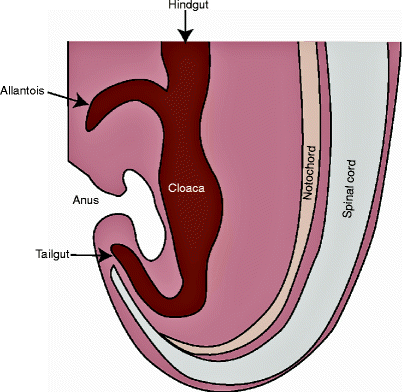

Fig. 50.2
The embryonic postanal gut. At 35 days of gestation, the tailgut extends caudal to the cloaca. Persistence of the postanal gut produces a tailgut cyst
The etiology of retrorectal teratomas is disputed. Presacral teratomas are thought to emerge from pluripotential embryonic rests positioned in the perirectal tissue [10, 24, 25]. Hannon et al. [10] believed that this tissue derives from Hensen’s node. Larsen [26, 27] and Coco and colleagues [26, 27] specify that these primordial germ cells err on their usual path from the yolk sac along the dorsal body wall to the gonads between the fourth and sixth weeks of gestation.
The embryologic defect that produces rectal duplications is a subject of debate. A number of theories have been advanced, none of which adequately explain the various types of duplication [28]. It has been suggested that these lesions represent the persistence of the usually ephemeral intestinal diverticuli that appear during the eighth or ninth week of embryogenesis [29, 30]. However, later authors point out that these buds are located on the antimesenteric surface of the intestine and incorporate only mucosa and serosa, not qualifying them as duplications. Moreover, these embryonic diverticuli are not a feature of the large intestine [31]. An abnormality during the recanalization of the occluded intestine in the sixth week of gestation was offered as an etiology for the creation of two lumens [29–32]. Furthermore, Veeneklaas [33] has commented that a defect in the separation of the embryonic notochord from the underlying endoderm may draw a portion of the primitive intestine dorsally, where it evolves independent of the remaining gastrointestinal tract. Finally, Edwards [34] has proposed that the duplication of all hindgut derivatives begins well before the formation of the primitive gut tube.
Epidermoid cysts are thought to arise from a defect in the closure of the ectodermal tube [35]. However, various authors ascribe epidermoid cysts to a displaced ectodermal remnant [36, 37]. Dermoid cysts are generated from caudal embryonic remnants [27]. Hannon and colleagues [10] state that these cysts develop because of an error in the fusion of the ectoderm, leading to the entrapment of the embryonic tissue in the space. The persistence of the urogenital “apparatus” also has been proposed as the source of a dermoid cyst [38]. Similarly, dermoid cysts have been attributed to a failure of the coccygeal vestige of the neural canal – the fovea coccygea– to regress [39]. Chordomas emerge from a persistent primitive notochord [10]. The mesodermic notochord normally develops in the third week of gestation but ultimately regresses, forming the cartilage and the vertebral bodies [10].
Classification
Various methods to classify retrorectal tumors have been proposed. Although they share a common location, these heterogeneous tumors differ in their pathology and origins. Traditionally, the system suggested by Lovelady and Dockerty [40] in 1949 has been the prototype, dividing these lesions into congenital, inflammatory, neurogenic, osseous, soft-tissue, and miscellaneous tumors. Uhlig and Johnson [2] condensed the system by including the soft-tissue tumors in the miscellaneous division (Table 50.1). Wolpert et al. [9] prefer to eliminate the inflammatory lesions. A simpler categorization is offered by Lev-Chelouche and colleagues [41] and includes benign congenital, malignant congenital, benign acquired, and malignant acquired tumors. These authors state that the tumors in these groupings, although composed of varied pathologic subtypes, have a similar presentation, treatment, and prognosis, and although this arrangement does not alter the management of these tumors, it may be better understood by patients. Chêne and Voitellier [24] further simplify the classification into developmental cysts, teratomas, chordomas, and nonembryonic tumors.
Table 50.1
Classification of retrorectal tumors
Congenital tumors |
Developmental tumors |
Tailgut cyst (cystic mucinous hamartoma) |
Teratoma |
Teratocarcinoma |
Rectal duplication |
Epidermoid cyst |
Dermoid cyst |
Anterior sacral meningocele |
Chordoma |
Neurogenic tumors |
Ependymomas |
Ganglioneuroblastoma |
Ganglioneuroma |
Malignant schwannoma |
Neuroblastoma |
Neurofibroma |
Neurofibrosarcoma |
Neurolemmoma |
Schwannoma |
Osseous tumors |
Aneurysmal bone cyst |
Chondroma |
Chondromyxosarcoma |
Ewing’s sarcoma |
Giant cell tumor |
Osteochondroma |
Osteogenic sarcoma |
Osteoma |
Simple bone cyst |
Miscellaneous tumors |
Arteriovenous malformations |
Carcinoid tumor |
Extra-abdominal desmoid tumor |
Fibroma |
Fibrosarcoma |
Hemangioendothelioma |
Hemangioma |
Hemangiopericytoma |
Leiomyoma |
Lipoma |
Lymphoma |
Metastatic tumors (e.g., breast and prostate cancer) |
Myelolipoma |
Multiple myeloma |
Soft tissue sarcoma |
Inflammatory tumors |
Abscess (perirectal, pelvic) |
Complicated diverticulitis |
Crohn’s disease |
Foreign body |
Foreign body granuloma (e.g., mineral oil, barium) |
Internal fistula |
Incidence
Retrorectal tumors are rarely found in the adult population. The majority of the observations of these tumors are gathered from case reports or small series. The overall incidence of these tumors ranges from 0.0025 to 0.015 % [1]. Larger series from tertiary care centers calculate that one retrorectal tumor is diagnosed for every 40,000 to 63,000 admissions (Table 50.2) [45, 55, 56]. Spencer and Jackman [57] identified retrorectal lesions in 0.02 % of their routine proctosigmoidoscopies over a 1 year period, for 3 tumors out of 20,851 examinations. Various reviews suggest a frequency of 1.4–6.5 cases per year in the U.S. [27]. Because these figures represent the experience of referral centers, the incidence of these tumors may be under-represented [58]. Uhlig and Johnson [2] estimated that, in a metropolitan area, approximately two retrorectal tumors will be diagnosed each year. In some rare instances, two presacral tumors may coincide; Krivokapic et al. [59] described a 37-year-old woman with an enteric cyst and an anterior sacral meningocele. Other retrorectal masses that may be discovered concurrently with an anterior sacral meningocele include teratomas, epidermoid cysts, lipomas, and teratocarcinomas [45, 59]. Most of these retrorectal tumors, when diagnosed in adults, present during middle age (see Table 50.2). Diverse series record a range of age at operation between 29 and 60 years old, with the majority of the patients in their 40s [2]. Retrorectal tumors are 2–15 times more common in women [35, 45, 60]. Of the retrorectal tumors in the series from Uhlig and Johnson [2], 73 % were in women. Aslan [35] comments that an average surgeon will detect one retrorectal tumor during his or her career, and that patient most likely will be a woman. The greater incidence of these tumors in women during their childbearing years, particularly in the peripartum period, may be linked to the frequent routine pelvic examinations performed, rather than an actual sex disparity [4, 61]. Three to thirty percent of retrorectal tumors are diagnosed in pregnant women [4]. In the audit from Uhlig and Johnson, of the 24 women of childbearing age, seven were pregnant or postpartum at the time of diagnosis.
Table 50.2
Demographic reports of retrorectal tumors
Duration (years) | Cases | Average age (years) | Female | Malignancy | Malignancy (male) | |
|---|---|---|---|---|---|---|
Freier et al. [42] (1971) | 35 | 21 | NR | 12 (57 %) | 12 (57 %) | NR |
Uhlig and Johnson [2] (1975) | 30 | 63 | NR | 46 (73 %) | 26 (42 %) | NR |
Localio et al. [43] (1979) | 15 | 20 | 45 | 10 (50 %) | 12 (60 %) | 9 (75 %) |
Cody et al. [44] (1981) | 28 | 39 | NR | 21 (53.8 %) | 39 (100 %) | 18 (46 %) |
Jao et al. [45] (1985) | 19 | 120 (100 adults) | 43 | 74 (61.6 %) | 51 (43 %) | 33 (65 %) |
Hjermstad and Helwig [20] (1987) | 35 | 53 | 35 | 41 (77 %) | 1 (2 %) | NR |
Lee and Symmonds [46] (1988) | 15 | 70 | NR | 70 (100 %) | 21 (30 %) | 0 (0 %) |
Böhm et al. [1] (1993) | 12 | 24 | 29 (DC) | 19 (79 %) | 4 (17 %) | 2 (50 %) |
51 (C) | ||||||
Wang et al. [47] (1995) | 14 | 45 | 41.1 | 25 (55.5 %) | 22 (48 %) | 20 (91 %) |
Pidala et al. [48] (1999) | 20 | 14 | 44 | 12 (85.7 %) | 1 (7 %) | NR |
Lev-Chelouche et al. [41] (2003) | 10 | 42 | 40.6 | 28 (67 %) | 21 (50 %) | 11 (52 %) |
Smith et al. [49] (2004) | 23 | 43 | 49 | 26 (60 %) | 9 (39 %) | NR |
Glasgow et al. [50] (2005) | 22 | 34 | 48 | 21 (62 %) | 7 (21 %) | 6 (86 %) |
Buchs et al. [51] (2007) | 9 | 16 | 37 | 13 (81 %) | 0 (0 %) | 0 (0 %) |
Woodfield et al. [16] (2007) | 8 | 27 | 30 (B) | 17 (63 %) | 7 (26 %) | 4 (57 %) |
60 (M) | ||||||
Grandjean et al. [52] (2008) | 15 | 30 | 43 | 23 (58 %) | 1 (3 %) | NR |
Pappalardo et al. [53] (2009) | 14 | 34 | 42 | 19 (56 %) | 14 (41 %) | NR |
Mathis et al. [54] (2010) | 23 | 31 | 52 | 28 (90 %) | 4 (13 %) | 0 (0 %) |
Congenital tumors represent 55–81 % of retrorectal tumors [2, 27, 41, 45, 48]. In contrast, Lev-Chelouche and colleagues [41] observed an equal incidence of all tumor types. The most common of the congenital tumors, comprising 60 % of such lesions, are the developmental cysts, including tailgut cysts, teratomas, teratocarcinomas, rectal duplications, epidermoid cysts, and dermoid cysts [8, 36]. In the series from Jao et al. [45], the average age of diagnosis was 33 years. The developmental cystic lesions are recognized mainly in women during middle-age, principally in their 30s to 50s [2, 23, 62]. Various studies indicate a female:male predominance for developmental cysts of 3:1, although Jao and colleagues [45] detected a greater discrepancy of 15:1 [20, 62]. Among the large series, the incidence of the retrorectal developmental cystic lesions in women reaches 85 % [2, 48].
Approximately 50 % of these developmental cysts are tailgut cysts, also known as cystic hamartomas or mucus-secreting cysts (Table 50.3) [56]. Uhlig and Johnson [2] recorded 16 tailgut cysts (62 %) among the benign developmental cysts in their review of 63 patients, and 93.7 % of these tailgut cysts were in women. In an audit of tailgut cysts from Hjermstad and Helwig [20], the majority (77 %) appeared in women. Conversely, Hannon and colleagues [10] discovered tailgut cysts more commonly in men. Hjermstad and Helwig noted an average age of 35 years amongst 53 patients with a tailgut cyst; although the age distribution for the female patients conformed to a bell curve, the authors did not identify a similar age peak in their three male patients.
Table 50.3
Pathology of retrorectal tumors
Localio et al. [43] | Jao et al. [45] | Lee and Symmonds [46] | Böhm et al. [1] | Lev-Chelouche et al. [41] | Glasgow et al. [50] | Pappalardo et al. [53] | |
|---|---|---|---|---|---|---|---|
Tailgut cyst | 0 | 16 | 0 | 6 | 12 | 2 | 3 |
Epidermoid cyst | 0 | 15 | 5 | 5 | 0 | 5a | 3 |
Dermoid cyst | 1 | 0 | 0 | 0 | 0 | – | 1 |
Teratoma | 3 | 15 | 24 | 9 | 0 | 8 | 2 |
Teratocarcinoma | 0 | 3 | 4 | 0 | 0 | 1 | 1 |
Rectal duplication | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
Anterior sacral meningocele | 0 | 2 | 1 | 0 | 0 | 0 | 3 |
Chordoma | 8 | 30 | 0 | 4 | 9 | 3 | 5 |
Neurogenic | 1 | 14 | 16 | 0 | 4 | 6 | 1 |
Osseous | 3 | 13 | 10 | 0 | 3 | 2 | 3 |
Inflammatory | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
Miscellaneous | 2 | 14 | 10 | 0 | 14 | 7 | 7 |
Total | 20 | 120 | 70 | 24 | 42 | 34 | 34 |
Teratomas are rare in the adult population. The large series indicate an incidence of 0–37.5 % (see Table 50.3). These tumors are more commonly diagnosed in infants and children and are found in 1 per 30,000 to 43,000 live births [23, 62]. Among adults, these masses, as with other developmental cysts, are recognized primarily in middle-aged women [6, 62]. Uhlig and Johnson [2] reported only two teratomas in a study of 63 patients treated over a 30-year period. The review from Jao et al. [45] recorded seven teratomas (7 %) and one teratocarcinoma (1 %) among their adult patients.
Rectal duplications are similarly unusual in adults. These malformations are more frequently diagnosed in children younger than 12 years of age [25, 30]. Pappalardo and colleagues [53] detected two rectal duplications (5.8 %) in their series of 34 patients. These tumors are more prevalent among women. Most intestinal duplications lie outside of the rectum. Of the 315 cases of intestinal duplications noted in the literature by 1951, only 10 (3.2 %) involved the rectum [28, 63, 64]. Rectal duplications account for between 1 and 8 % of all gastrointestinal duplications [25, 32, 62, 65].
Approximately 15 % of retrorectal tumors are epidermoid cysts [35, 45, 50]. Jao et al. [45] diagnosed epidermoid cysts in 12.5 % of their patients. Dermoid cysts in the retrorectal space represent a small percentage of all dermoid cysts [25]. The large reviews indicate that dermoid cysts account for 0–5 % of retrorectal masses [43, 45, 53].
Anterior sacral meningoceles are rare congenital cystic tumors. By 2007, only 240 case reports were cited in the literature since its initial description in 1837 [59, 66, 67]. Women account for 85 % of these patients [45, 59]. Jao and colleagues [45] identified two cases (1.7 %) of anterior sacral meningocele, both in women, in their series of 120 patients. Some anterior sacral meningoceles demonstrate an autosomal dominant inheritance as part of the Currarino syndrome [45, 62]. These lesions also have been associated with Marfan’s syndrome [66]. These masses are further affiliated with abnormalities such as spina bifida; tethered cord; spinal dysraphism (rachischisis); bicornuate uterus; duplications of the rectum, uterus, or vagina; and anal stenosis or imperforate anus [8, 59, 62].
Inflammatory tumors comprise about 5 % of retrorectal tumors [2]. Hannon et al. [10] suggest, however, that such lesions are actually the most common of the retrorectal tumors. Inflammatory retrorectal “tumors” are acquired pathologies that arise from various sources [68]. Uhlig and Johnson [2] recount patients with such masses due to hemorrhoidal sclerotherapy with phenol and mineral oil, occult perforated diverticulitis, and one case of an abscess after a subtotal colectomy for ulcerative colitis. Retrorectal tumors with a neurogenic or osseous origin represent 10 and 5–10 % of these lesions, respectively [58]. Ten to twenty-five percent of retrorectal tumors generally are included in the miscellaneous category [8, 58].
Malignant Retrorectal Tumors
Malignant tumors represent a small proportion of retrorectal tumors. Overall, one of three retrorectal tumors are determined to be malignant in either sex [2]. Guillem and colleagues [60] comment that 30–50 % of retrorectal tumors are malignant (see Table 50.2). Uhlig and Johnson [2] found a 42 % incidence of malignancy in their series of 63 patients. In contrast, Spencer and Jackman [57] identified malignant tumors in 8.7 % of their patients. Older patients are more likely to harbor a malignant retrorectal tumor than younger patients, with an average age of 60 and 30 years, respectively [16]. Similarly, Glasgow et al. [50] detailed a significant discrepancy in the age of diagnosis: 63 years and 43 years for malignant and benign tumors, respectively (P = 0.003). Malignancy is more common among men, as was the case in the review from Jao and colleagues [45], accounting for 64.7 % of malignant tumors (see Table 50.2). In the audit from Glasgow et al., this sex difference was significant: 86 % of the malignancies were in men (P = 0.007). However, Singer and colleagues [61] remark that malignancy arises equally between the sexes. Tumor size does not correlate with malignancy [10], yet Wang et al. [47] recorded a significant difference in the mean size of malignant and benign retrorectal tumors: 16.5 cm (range, 5–40 cm) and 6.3 cm (range, 1.5–20 cm; P < 0.05), respectively.
The most common retrorectal malignancy is the chordoma [10, 44, 61]. Among 39 malignant retrorectal tumors treated by Cody and colleagues [44], the majority (38 %) were chordomas. Yet Uhlig and Johnson [2] noted that these retrorectal tumors numbered only six (9.5 %) in their series. For chordomas, the average age of presentation was in the 60s and 70s in the audit from Freier et al. [8, 42], where they reported that a diagnosis earlier than the age of 30 years is unusual [8]. Overall, men are diagnosed with a chordoma more frequently than women by a ratio of 2:1 to 5:1, in contrast to the experience of Uhlig and Johnson [2], where five women but no men had this tumor [10, 45, 58]. The incidence of metastases from chordomas is as high as 20 % [16]. When metastatic, the tumor spreads to the lymph nodes, bone, lungs, and liver [1]. In the audit from Jao and colleagues [45], metastatic disease was recognized in 17 % of patients with a chordoma: lung (n = 2); liver (n = 1); rib, femur, and lumbar spine (n = 1); and thoracic spine (n = 1).
Malignant cystic retrorectal tumors are infrequent. Solid tumors are more commonly malignant than cystic masses (60 and 10 %, respectively) [23, 58]. Among various studies, the incidence of malignant degeneration of cystic retrorectal tumors ranges from 10 to 60 % [61]. Chêne and Voitellier [24] comment that most cases of malignant cystic degeneration occur in men.
Tailgut cysts are rarely associated with a malignancy [20]. Series have suggested a 2 % incidence of malignancy in these cystic lesions [20, 54]. Mathis et al. [54] identified four malignant tailgut cysts (13 %) – three adenocarcinomas and one carcinoid tumor – in women aged 31–79 years. They determined that a malignancy was not affiliated with patient age, symptom duration, tumor size, or a palpable mass [54]. Carcinoid tumor, adenocarcinoma, sarcoma, and adenosquamous carcinoma all have been reported in tailgut cysts [54, 69–73]. In a case study from Krivokapic and colleagues [74], a 47-year-old woman was diagnosed with a tailgut cyst containing an adenosquamous carcinoma; the tumor was initially identified at the time of a hysterectomy that was performed 11 years before the resection. Pseudomyxoma peritonei has been described after the removal of a tailgut cyst with a well-differentiated focus of mucinous adenocarcinoma [75]. An adjacent rectal cancer may invade a tailgut cyst via an anal fistula, as was chronicled in a 32-year-old man by Yamaguchi et al. [76].
Teratocarcinoma is unusual in adults. Malignant degeneration occurs in approximately 1–10 % of teratomas in this cohort [2, 25, 29, 58]. Jao and colleagues [45] recorded a rate of malignancy of 12.5 % in their adult patients with teratoma. In contrast, in pediatric patients, malignancy becomes more common with older age, but incidence declines after the second decade of life [10, 27, 58]. Malignant changes in the adult population primarily are associated with the immature and not the mature type of teratoma [10]. Teratocarcinomas are larger than their benign counterparts, predominantly comprising solid and not cystic elements [10]. The review from Uhlig and Johnson [2] reported two patients with a teratocarcinoma, a 70-year-old woman and a 49-year-old woman, the former with a thyroid follicular carcinoma and the latter with an embryonal carcinoma arising from respiratory epithelium. The majority of these malignancies feature adenocarcinoma or endodermal sinus tumors [10].
Malignancy rarely has been found in a rectal duplication [30]. Eighteen percent of adult rectal duplications are malignant [77]. These cases of adenocarcinoma were discovered in adults older than 30 years of age [3]. Orr and Edwards [78] identified an adenocarcinoma in a cystic rectal duplication, and the patient died 8 months after excision. Yang et al. [37] suggest that the malignant degeneration of an epidermoid cyst may result from chronic inflammation of the cyst wall. Similar instances of malignancy in epidermoid cysts have been reported in the skin, liver, and brain [37]. For epidermoid cysts of the brain, malignancy develops between 3 months to 33 years (average, 8.4 ± 11.3 years) after the initial diagnosis of the benign lesion [37]. A case of an epidermoid cyst with associated squamous cell carcinoma was described by Yang and colleagues in a 63-year-old woman with complaints only of acute urinary retention. Thway et al. [25] reported a 64-year-old man with a dermoid cyst, the apocrine glands of whom were affected by extramammary Paget’s disease. A 45-year-old man with a dermoid cyst containing extramammary Paget’s disease was described by Roy et al. [79].
Pathology
Retrorectal tumors include masses of heterogeneous pathology. The cystic tumors account for approximately 40.8 % of these masses [68]. However, solid masses and inflammatory processes may develop cystic changes that further confound the diagnosis [55]. The pathology of the more common retrorectal tumors is described, including tailgut cyst, teratoma and teratocarcinoma, rectal duplication, epidermoid and dermoid cysts, anterior sacral meningocele, chordoma, and schwannoma.
A tailgut cyst is usually multiloculated. In the series from Hjermstad and Helwig [20], half of the cysts were grossly unilocular, but 81 % were microscopically multicystic. The lesion is comprised of one to two “parent cysts” and a variable number of “daughter cysts” [2]. The epithelium ranges from squamous, columnar, and ciliated columnar to transitional [2, 10]. Squamous epithelium was identified most frequently in the review from Hjermstad and Helwig. Although disorganized smooth muscle fibers may be found in the cyst wall, there is no complete muscular coat, myenteric plexus, or serosa, as in a rectal duplication [10, 20]. These lesions do not incorporate dermal appendages (unlike dermoid cysts), or intestinal epithelium (differentiating them from rectal duplications), or, for that matter, all three germ layers (contrasting them with teratomas) [61]. These thin-walled cysts contain a serous to clear, yellow, or light green mucus to a thick brown paste [2, 20, 80]. Although the cyst may closely adhere to the posterior rectal wall, there is no actual communication with the rectum unless it is created iatrogenically [20]. Inflammation (or infection), associated with approximately 50 % of the tailgut cysts in the audit from Hjermstad and Helwig, likely produces this intimate attachment. The tumor is confined to the retrorectal space but may infrequently extend superior, lateral, or anterior to the rectum [20]. Rare cases of a prerectal location for these cysts have been reported [81].
Teratomas are formed from tissue foreign to the retrorectal space [27]. These tumors are classified by their extent as intrapelvic, extrapelvic, or both. Primarily seen in the pediatric population, the tumor may grow beyond the boundaries of the pelvis (types I–III) and even extradurally to the spinal cord [10]. Grossly, teratomas may be cystic or solid. Coco and colleagues [27] described the teratoma as an encapsulated, white, globular lesion. Cystic teratomas are lined by various epithelia including squamous, cuboidal, or ciliated columnar [10]. The cystic portions enclose a keratinous or serous fluid [27]. The solid material includes the derivatives of all three germ layers, represented by endoderm (e.g., respiratory epithelium), mesoderm (e.g., cartilage and fat), and ectoderm (e.g., epidermis and skin appendages) [27]. At least two of these germ cell layers must be present for the diagnosis of teratoma [26]. These tissues may be organized to mimic diverse organs [26]. Teratomas are categorized as mature, immature, or malignant (teratocarcinoma) based on their degree of differentiation [23]. The teratoma is characteristically attached to the coccyx, leading some authors to recommend a routine coccygectomy as part of the treatment for these tumors [8, 10].
Duplications are developmental malformations that may arise at any point along the gastrointestinal tract, from the esophagus to the anus. The majority (94 %) of these duplications are hollow cystic structures, also known as enteric or enterogenous cysts [28, 29]. Tubular-shaped duplications replicate short or long segments of the intestine, often in conjunction with a urinary tract duplication or sacral abnormalities [28, 64]. Whereas most alimentary duplications (80 %) do not fistulate to the intestine, the giant diverticulum does feature such a communication [28, 31, 32]. Of the 70 cases of rectal duplications recorded in the literature by 1962, 54.3 % were tubular and 45.7 % were cystic [29, 31]. An alimentary duplication is affixed to the portion of the gastrointestinal tract with which it is associated, usually attaching to the mesenteric surface and sharing the same vascular supply [25, 29, 30]. A gastrointestinal duplication is characterized by its adherence to a section of the gastrointestinal tract; its complete (usually double layered), smooth-muscle sheath; and its mucosa composed of cells typical of the gastrointestinal tract [3, 28, 30, 32]. However, some gastrointestinal duplications contain mucosal cells not generally found in the adjacent bowel; La Quaglia and colleagues [3, 28, 30, 32, 80] identified gastric, colonic, transitional, squamous, and urothelial cells within 11 rectal duplications [28, 30, 32, 80]. A ciliated epithelium rarely has been discovered in these lesions [32]. Multiple types of epithelia may coexist in one lesion [29]. In some cases, ectopic gastric, pancreatic, or urothelial mucosa may be detected [62]. The alimentary duplication incorporates all three intestinal layers found in the normal gastrointestinal tract, namely, mucosa, muscularis propria, and serosa [30]. Rarely, one patient may have two duplications [32]. Diminutive cysts may closely accompany a duplication, predisposing a patient to a recurrence if not recognized and removed [29]. Alavanja et al. [30] described a rectal duplication in a 60-year-old woman as grossly consisting of a soft, fibrous tissue with the inner lining similar to mucosa; the microscopic examination demonstrated a urothelial-type epithelium, an inflamed submucosa, and striated muscle. A rectal duplication in a 39-year-old women treated by Monek and colleagues [32] revealed “heterotopic cylindric ciliated epithelium with mucin-secreting cells, connective tissue, and striated muscle.” In contrast to complete twinning of the hindgut, isolated rectal duplications have no association with genitourinary abnormalities [29].
Epidermoid cysts are grossly firm yet elastic, well-encapsulated lesions [82]. Ueda et al. [82] comment that epidermoid cysts exhibit a poor vascular supply. The thin-walled, unilocular epidermoid cysts are composed of a stratified squamous epithelium with keratohyaline granules [36, 62, 82]. The usually clear cyst fluid includes water, keratin, cholesterol, and desquamated epithelium [36]. In contrast, Sasaki and colleagues [56] found a “cheese-like material.” Dermoid cysts, although also lined by a stratified squamous epithelium, involve skin appendages such as hair follicles, sweat glands, and calcified materials (i.e., tooth buds) (Fig. 50.3) [4, 62]. These often multilocular lesions almost universally contain a keratinoid or sebaceous fluid, although others [27, 62, 80] noted a “muddy material.” A fatty substance is reported in 67–75 % of these lesions and calcifications are reported in 31 % [62, 80].
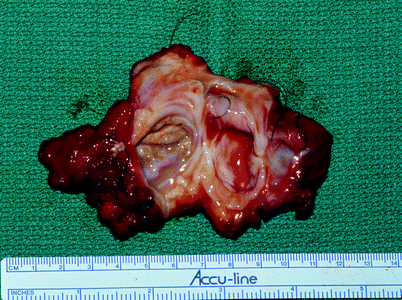

Fig. 50.3
Epidermoid presacral cyst. This recurrent dermoid cyst was removed from a 39-year-old woman via a Kraske approach
An anterior sacral meningocele comprises the herniation of the dural and arachnoid membranes of the spinal cord through a defect in the ventral surface of the sacrum [59]. The herniated sac is continuous with the subdural space [58]. This congenital unilocular cystic lesion is filled with clear, watery cerebrospinal fluid [59]. Chordomas, derived from the primitive notochord, are composed of a gelatinous material (Fig. 50.4) [10]. These lobulated, slow-growing tumors are locally invasive and destructive but not usually metastatic [10, 58]. The sacrococcygeal region is the most common site for a chordoma (30–50 %), although the tumors may occur at any point along the spine [6]. Schwannomas are the most prevalent neurological tumor [23]. These tumors comprise fascicular bundles of spindle cells within a capsule of epineurium [23]. Degenerative changes such as fibrosis, calcifications, hemorrhage, and cyst development often are evident [23].
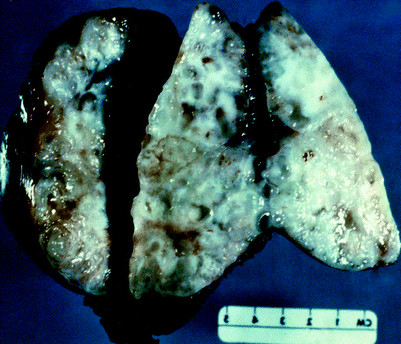

Fig. 50.4
Presacral chordoma. A transsacral procedure was employed to remove this recurrent chordoma from a 35-year-old man
Symptoms
The majority of retrorectal tumors are asymptomatic. Pidala et al. [48] noted that 57 % of their 14 patients reported no symptoms attributable to the tumor. An absence of symptoms was recorded by Lev-Chelouche and colleagues [41] in only 26 % of their patients. Symptoms due to these overall slow-growing lesions are vague, making the diagnosis moderately difficult. Singer et al. [61] reported an average symptom duration of 4.9 years before diagnosis, with a range of 1–11 years. Similarly, the mean length for symptoms was 3.7 years in the series from Uhlig and Johnson [2]. An acute presentation is rare [20]. Complaints generally arise related to the mass effect from the tumor, depending on its size, location, and extent [4, 9, 82]. The presence of infection, malignancy, or necrosis of the tumor usually is associated with symptoms. Pidala and colleagues [2, 48, 61, 82] found that 50 % of their patients with symptoms had an infected retrorectal cyst, which was also noted by other groups. [2, 61, 82]. Invasion of adjacent structures, especially of nerves, also produces symptoms [79]. Nerve root involvement is suggested by radicular pain, urinary or fecal incontinence (or both), or sensory or motor deficits [43]. Among the symptoms frequently identified by patients are anal, rectal, perineal, or pelvic pain or pressure; constipation, difficult or painful defecation, and narrowed or “ridged” stools [27, 30, 43, 59, 61, 83].
Pain – the most common complaint – is usually dull and difficult to localize [48, 61]. Among symptomatic patients with a tailgut cyst in the review from Hjermstad and Helwig [20], 82 % described pain in the rectum or lower back, and seven of these patients (39 %) specified that the pain was exacerbated by defecation [20]. Chordoma especially is known for causing painful defecation, although the tumor also is associated with constant rectal pain [10, 84]. In some patients, rectal pain is exacerbated by sitting, as was related by MacLeod and Purves [31] in a 39-year-old woman with a rectal duplication, who also felt a lump over her coccyx. In other patients, lower back pain may coincide with ambulation [61]. Localio and colleagues [43] recorded lower back or sacral pain in 18 patients (90 %), with a median duration of 12 months (range, 2 days to 8 years). The lower back pain attributable to a chordoma is often postural or worsened by sitting [45, 61]. In the event of invasion of the sacral plexus, nebulous pains may be experienced in the lower back or buttocks, with radiation down the legs [10, 61]. Chen et al. [36] commented that abdominal pain may result from a spontaneous rupture of a cystic lesion, as in their 62-year-old patient with a large retrorectal epidermoid tumor and constant, dull pain in the right lower quadrant.
Some patients recall trauma to the coccyx before the diagnosis of a retrorectal tumor [2]. Symptoms developed subsequent to coccygeal trauma in 15 % of patients with a malignant retrorectal tumor in the series from Cody and colleagues [44] and in 11 % with a retrorectal mass in the review from Jao et al. [45]. Sasaki and colleagues [56] suggested that trauma directly caused such lesions as an epidermoid or dermoid cyst; however, the trauma most likely brings attention to a previously asymptomatic tumor [44]. Hjermstad and Helwig [20] note that cyst rupture secondary to trauma may promote an inflammatory reaction, producing the symptoms that lead to the diagnosis of a retrorectal tumor.
Bowel symptoms are common in patients with a retrorectal tumor. Such complaints were reported by 31 % of the female patients in the series from Lee and Symmonds [46] and 44 % of the patients in the review from Wang et al. [47]. The alterations in bowel habits arise because of changes in the angulation of the rectum, impingement upon the rectal lumen, or both [58]. Fecal incontinence may result from nerve involvement [61].
Urinary symptoms are often ascribed to retrorectal tumors. Lee and Symmonds [46] attribute these urinary, as well as bowel, complaints to the size and not the pathology of the tumor. Nerve involvement of the tumor may also produce these urinary symptoms [61]. Ten percent of the patients in the series from Lee and Symmonds and 13 % in the review from Wang and colleagues [46, 47] described urinary retention, urinary incontinence, and urgency. Abnormally frequent micturation has been associated with retrorectal tumors [20, 26, 43]. Thway et al. [25] recounted one 64-year-old man with a dermoid cyst containing extramammary Paget’s disease and noted only polyuria as the main presenting feature. The patient detailed by Chen and colleagues [36] developed obstructive uropathy, demonstrating right hydronephrosis and hydroureter due to the retrorectal tumor. Similarly, in a case report from Yang et al. [36], a 63-year-old woman presented with acute urinary retention and suprapubic pain as a result of a 17 × 11 × 10 cm cystic retrorectal tumor that impinged upon the bladder, uterus, and rectum.
Painless rectal bleeding may occur secondary to a retrorectal tumor. One patient required a blood transfusion because of “massive proctorrhagia,” whereas two patients had chronic bleeding per rectumin the review from Grandjean and colleagues [20, 52]. Rectal bleeding, mucoid anal drainage, or both were experienced by four patients with developmental cysts in the series from Wang et al. [47]. Most cases of rectal duplication become symptomatic early in life. A cystic rectal duplication produces pain because of its dilatation with mucus [30, 64]. Ectopic mucosa may cause bleeding, as in a report of a 4-year-old girl with ectopic/heterotopic gastric mucosa in a giant diverticulum of the rectum, generating a rectal ulcer [28, 30]. Bleeding may also result from pressure necrosis upon the rectum from a dilated duplication [64, 78]. Bowel obstruction arising from a rectal duplication also has been encountered, although this is less common than with duplications elsewhere in the alimentary tract because of the large capacity of the rectum [3, 31]. Furthermore, in rare cases, the rectal duplication may prolapse per the rectum [3]. La Quaglia and colleagues [3] noted that infection frequently accompanies a rectal duplication. However, many of these patients initially had a suspected perianal abscess drained, thus introducing bacteria into a previously sterile mass [3]. Before surgery, a 39-year-old female patient with a cystic lesion, which later was determined to be a rectal duplication, underwent aspiration and then incision and drainage, yielding a large amount of purulent fluid; as a consequence, she developed a draining sinus tract at the incision site [31]. Rectal duplications with a fistula to the posterior midline may demonstrate drainage of a mucoid or purulent material [3].
Gynecologic complaints have been ascribed to retrorectal tumors. A 27-year-old woman reported vaginal swelling and difficult defecation in conjunction with posterior vaginal wall prolapse, thought to be consistent with a grade III rectocele, but ultimately this was determined to be a result of a retrorectal epidermoid cyst [35]. Patients may also recount irregular menstruation and dyspareunia [10, 61]. Retrorectal tumors may also complicate pregnancy. As of 1996, only 15 cases of such pregnancy complications had been recorded [40]. In the series from Lovelady and Dockerty [4], 7.8 % of the 127 retrorectal tumors were affiliated with obstetric complications. Pelvic dystocia may arise because of obstruction of the vaginal canal during delivery [4, 27]. However, small retrorectal tumors may permit vaginal delivery [59]. Uhlig and Johnson [2] described three women with a small retrorectal tumor that only became symptomatic because of an infection after a successful vaginal delivery; however, a large lesion usually mandates a Caesarean section. Krivokapic et al. [59] detailed a 37-year-old woman who underwent a Cesarean section because of an anterior sacral meningocele and enteric cyst discovered during her prenatal examinations. Yet, even a Caesarean section may be compromised by a large tumor. Sobrado and colleagues [59] chronicled a 25-year-old woman with a “huge” presacral tumor that impeded an emergency Cesarean section for hemorrhage from a placental abruption, resulting in the death of the fetus. Resection of the tumor may be attempted during pregnancy, as was done for a woman with a ganglioneuroma during her fourth month of pregnancy [2, 4]. However, at term, the increased blood flow within the pelvis renders a resection more treacherous [85].
Anterior sacral meningoceles present with headaches in one third of patients [59]. These headaches, along with nausea, may be precipitated by coughing, straining, defecation, squatting, or coitus [45, 59]. As the neck of the cyst is compressed by one of these maneuvers, the influx of cerebrospinal fluid into the meningocele is prevented, leading to a rise in intracranial pressure [59]. This elevation in intracranial pressure triggers the pain receptors within the posterior fossa, causing headache. Meningitis may arise in association with retrorectal tumors. Recurrent meningitis principally suggests a diagnosis of anterior sacral meningocele [9, 58]. A spontaneous microperforation may produce meningitis in these tumors, yielding a mortality rate of 30 % [59]. Alternatively, an iatrogenic “perforation” of an anterior sacral meningocele, as with a biopsy, may induce meningitis and is associated with a mortality rate of almost 100 % [59]. A rectothecal fistula has been reported as a source of meningitis in patients with an anterior sacral meningocele, which, in a 48-year-old man, was attributed to a stercoral perforation [86, 87]. Meningitis also has been described after the rupture of a dermoid cyst into the subarachnoid space [10].
A retrorectal tumor easily is confused with other pathologies. Singer and colleagues [61] noted that their seven patients had been treated for other complaints, including perirectal abscess; posttraumatic, psychogenic, or postpartum pain; pilonidal sinus; presacral abscess; and fistula-in-ano, before the diagnosis of a retrorectal tumor was made. These patients had an average of 4.7 diagnostic and therapeutic procedures before the correct diagnosis was made. Repeated recurrences of a perianal abscess or fistula should also suggest the rare possibility of a retrorectal tumor [9]. A 39-year-old patient, who ultimately was found to have a rectal duplication, was initially thought to have a chronic retroperitoneal abscess along with a left ureteric duplication [32]. Localio et al. [43] encountered one patient with an infected retrorectal teratoma that was instead managed as a pelvic abscess. Repeated incision and drainage procedures produce an inflammatory reaction that renders a subsequent excision of the retrorectal tumor difficult [10]. A pilonidal sinus that appears distal to the coccyx should also signal a possible retrorectal tumor [10]. Furthermore, a recurrent or nonhealing pilonidal sinus may represent an underlying retrorectal tumor. A recurrent pilonidal sinus featuring two classic midline pits in the gluteal cleft in association with a tailgut cyst was described by Satyadas and colleagues [88]. A physician may misinterpret a large, retrorectal cystic tumor as seen on transabdominal ultrasound as an ovarian cyst or other abdominal pathology, a misdiagnosis that should be suspected especially if the laparoscopy or laparotomy is negative [89–91]. Similarly, Localio et al. identified one patient with a ruptured teratoma who suffered only from recurrent fevers and malaise.
The presence of a malignant retrorectal tumor is not predicted by specific symptomatology [10]. Rarely, these masses may be discovered incidentally. In the series from Cody and colleagues [44], two asymptomatic, malignant retrorectal tumors (5 %) were diagnosed after a routine examination. The majority of patients with a malignancy are symptomatic. Jao et al. [45] comment that a symptomatic retrorectal lesion is more suggestive of malignancy than an asymptomatic mass. This finding is echoed by Glasgow and colleagues [50], who stated that benign tumors are asymptomatic more frequently than malignant masses (56 % vs. 14 %, respectively; P = 0.09). Malignancy may be heralded by symptoms such as leg paraesthesia or weakness; however, benign lesions may also give rise to these same complaints [27]. Cody et al. recorded a 95 % incidence of symptoms among their 37 patients with diverse malignant retrorectal tumors. Patients with a malignancy most frequently report pain, as was the case in 67 % of the symptomatic patients in the series from Cody et al., including sacrococcygeal, buttock, low back, rectal, or radicular pain [36, 44]. Sacrococcygeal pain (83 %) was most commonly communicated by patients with a malignant retrorectal tumor in the review from Glasgow and colleagues. They determined that only sciatic or pelvic pain was significantly associated with a malignancy (P = 0.02). Eighty-eight percent of the patients with a malignancy in the audit from Jao et al. described pain, in contrast to 39 % of the patients with a benign tumor. The series from Localio and colleagues [43] identified radicular pain (from invasion of the sacral nerve roots) in 50 % of 12 malignant tumors. These authors also correlate fecal or urinary incontinence with malignancy: their two incontinent patients were found to have advanced malignancies [9]. These bowel and bladder complaints were less commonly observed in the review from Cody et al. [44]. The interval to the diagnosis of a malignant and a benign retrorectal tumor nonsignificantly varied from 6.1 to 18.8 months, respectively (P = 0.148) in the audit by Wang et al. [47].
Infection
Retrorectal cystic lesions frequently present because of a concurrent infection. An infection may occur primarily or as a secondary process [10]. Abel and colleagues [61, 85] determined that one-third of these cystic retrorectal tumors develop an infection [61]. In the series from Spencer and Jackman [44, 57], infected tumors were found in 31.6 % of patients undergoing surgery [44, 57]. The incidence of infected developmental cysts, particularly epidermoid and enteric cysts, is reported as 30–50 % by Dahan et al. [62]. Verazin and colleagues [92] attribute this association of retrorectal tumors with a primary infection to the relatively poor vascular supply to this area, their location adjacent to the rectum, and their susceptibility to trauma or biopsy. They further comment that bacteria such as the Clostridiumspp. are common in tumors that are necrotic or advanced [92]. Patients may experience intermittent fevers and/or night sweats or pelvic pain as a consequence of the infected lesion [61, 62, 85].
The Currarino Syndrome
A genetic basis for retrorectal tumors was first described as a syndrome in 1981 by Currarino [93]. The genetic mutation for this autosomal-dominant disorder has been localized to the homeobox gene HLXB9on chromosome 7q36 [94, 95]. In 50 % of cases, the syndrome is familial; the remainder are sporadic [93, 94]. The embryologic basis for the syndrome is uncertain. Currarino suggested the “split notochord” model in which early embryonic adhesions between the endo- and ectoderm lead to the characteristic findings of this syndrome [93, 94, 96]. This autosomal-dominant genetic disorder includes retrorectal tumors, anorectal defects (anorectal stenosis or a low imperforate anus), and anterior sacral abnormalities [62]. A myriad of retrorectal tumors may be involved in this syndrome, including anterior sacral meningocele, enteric cyst, tailgut cyst, dermoid cyst, or teratoma [25, 62]. The most common retrorectal masses in this syndrome are the anterior sacral meningocele and teratoma [96]. More than one retrorectal tumor may coexist [62]. Malignant degeneration of the retrorectal mass was identified in three children, all 2 years old, and four adults and included five teratomas, one leiomyosarcoma, and one neuroendocrine tumor [94]. The classic triad of retrorectal tumor, complete or partial sacral agenesis, and anorectal anomalies does not frequently occur concurrently [93]. By 1984, only 40 cases of a complete triad had been recorded [93]. In 80 % of cases, a complete Currarino triad is diagnosed by the age of 10 years; those patients with an incomplete Currarino syndrome are usually detected as adults [96]. The sacral defect is a constant finding [97]. Among the other abnormalities possibly evident in this syndrome are those of the kidneys, urinary tract, and gynecologic organs [25, 62]. A tethered spinal cord was found in 17 patients (58.6 %) in the review from Crétolle et al. [94]. Constipation is the most common complaint among these patients [62]; however, patients are asymptomatic in 33 % of cases [24, 94, 98]. Patients may present acutely with a bowel obstruction, perianal sepsis, or meningitis [95]. Family members should be screened for the syndrome with a sacral radiograph and genetic counseling should be offered [93].
Diagnosis
The correct diagnosis of a retrorectal tumor begins with the clinical suspicion that such a lesion is present. The identification of these masses initially relies upon the physical findings of an examination. The primary radiographic means to diagnose these lesions include endorectal ultrasound (ERUS), computerized tomography (CT), and magnetic resonance imaging (MRI). Among the other diagnostic modalities available are endoscopy, barium enema, angiography, and myelography, when appropriate.
A postanal dimple represents a connection between a retrorectal cystic lesion and the perianal skin. However, a clear tract between the postanal dimple and the cyst is only variably present [2]. The cone-shaped external opening occurs in the posterior midline, primarily over the perianal skin but possibly in the distal anal canal [3, 4]. The postanal dimple is distinguished from a fistula-in-ano by the absence of an internal opening at the dentate line [23]. However, a postanal dimple may coincide with a fistula-in-ano, as noted by Grandjean and colleagues [52], who treated two patients with a retrorectal tumor associated with both a postanal dimple and an intrasphincteric fistula-in-ano. Twenty percent of cystic rectal duplications demonstrate a postanal dimple [29]. In a series of 11 pediatric patients, La Quaglia et al. [52] identified such dimples in five children (45 %). These postanal dimples also are affiliated with 2–34 % of congenital lesions such as tailgut cysts and epidermoid cysts [1, 48, 61]. A postanal dimple was detected in 34.6 % of patients with a retrorectal cyst in the series from Uhlig and Johnson [2, 50] but in 5.8 % of retrorectal tumors in the review from Glasgow and colleagues [50]. Singer et al. [2, 50] detailed a well-epithelialized external opening that led to a retrorectal cystic lesion, a tailgut cyst, with persistent serosanginous or purulent drainage in an 18-year-old woman. The origin of the postanal dimple is uncertain. Hjermstad and Helwig [20] suggest that the dimple arises from traction on the postanal skin by the filum terminale during fetal development.
An external mass is encountered infrequently in adult patients. Primarily, extrapelvic extension of these masses, usually teratomas, is exhibited by neonates [6, 9]. Coco and colleagues [27] described a 40-year-old woman, with a history of a pelvic mass since birth, who presented with pelvic pain and a tender lesion on her right buttock, which later was determined to be a teratoma. The same authors also reported a 50-year-old man, diagnosed with a pelvic mass 25 years before presentation, with a palpable tumor on his right buttock, a dermoid cyst. A mass on the left buttock similarly was discovered in a 39-year-old woman with a rectal duplication [32]. Coccygeal abnormalities may be revealed during physical examination. Uhlig and Johnson [2] recorded six patients (9.5 %) with a deformed coccyx in addition to a retrorectal tumor.
The majority of retrorectal lesions are identified during digital rectal examination. Such evaluations often are performed in asymptomatic patients during routine physical examinations or prenatal assessments [80]. Jao et al. [45] diagnosed these tumors via a digital examination in 97 % of their patients. In contrast, only 35 % of the retrorectal tumors were palpated during digital examination in the audit from Glasgow and colleagues [50]. The index finger may distinguish a fixed mass or presacral fullness, usually in the midline but occasionally to its right or left [10]. In the series from Bellotti et al. [99], three retrorectal tumors were located in the posterolateral position. Because many of these tumors, particularly the cystic lesions, are soft and compressible, their presence may be overlooked. Various authors [2, 27, 61] emphasize that the examining digit must be maneuvered to feel the entirety of the retrorectal space. An epidermoid cyst is [68, 82] described as a firm, elastic, nontender mass during digital examination. An anterior sacral meningocele is suggested by a soft, fluctuant mass [59]. When the patient completes a Valsalva maneuver, an anterior sacral meningocele may enlarge (the “cry impulse”) during the digital examination [45]. Fullness and fixation in the retrorectal space implies an infected cyst [10]. Displacement of the rectum or anus or narrowing of the lumen as a result of the tumor may be evident [30, 65]. Sacral nerve invasion may be indicated by a patulous anus or hypoesthesia of the perineum [61]. A fistula may be discovered during the examination: a rectal fistula was detected during a digital rectal examination in four patients with a retrorectal tumor in the series from Mathis et al. [54]. A malignant lesion is not necessarily distinguished from a benign tumor by the digital rectal examination. Localio and colleagues [43] found that primary sacral malignancies were characterized by their fixation, yet this also was true of five benign tumors. Yang et al. [37] noted a “rubbery” retrorectal tumor that later was determined during digital examination to be an epidermoid cyst containing a squamous cell carcinoma. Chordomas were assessed to be primarily smooth, nontender, and rubbery [10, 43, 45], although two of the eight tumors in the review from Localio and colleagues were lobulated. In contrast, Lev-Chelouche et al. [41] commented that their patients’ chordomas were solid and fixed to the sacrum. Invasion of the sacrum may manifest with sacral tenderness during transrectal palpation [41].
Specific tumor markers may be elevated in some cases of retrorectal tumor. Coco and colleagues [27] identified a patient with a teratoma with a cancer antigen (CA) 125 of 109 ng/mL, although the levels of CA 15-3, CA 19-9, α-fetoprotein, and β–human chorionic gonadotropin were within normal limits. Teratomas feature a high carcinoembryonic antigen and α-fetoprotein level in 82 and 53 % of cases, respectively [45]. Chêne and Voitellier [24] commented that an isolated increase in the level of carcinoembryonic antigen (CEA) may indicate an epidermoid cyst. An elevated CA-125 of 103.4 U/mL (normal, <35 U/mL) was demonstrated in a 62-year-old woman with a epidermoid cyst that initially was thought to be an ovarian cancer [36]. An elevated level of CA 19-9 has been reported in association with a retrorectal tailgut cyst, but the level decreased to the normal range 1 month after resection [100]. A high tumor marker level only variably predicts a malignancy. In a 54-year-old woman described by Rogers et al. [71], malignancy in a recurrent, previously benign tailgut cyst was signaled by an elevated CEA level. An elevated serum α-fetoprotein also is associated with a teratocarcinoma [24, 45]. These tumor markers have an uncertain value for the postoperative surveillance of tumor recurrence. The recurrence of a tailgut cyst containing a poorly differentiated adenocarcinoma after surgery and radiation therapy was heralded by a rise in the levels of CEA and CA 19-9, both of which had been markedly elevated before treatment but then had normalized [101].
Flexible endoscopy or a rigid proctoscopy should be performed to rule out mucosal involvement of the rectum. These examinations generally reveal normal mucosa, possibly with a smooth indentation of the posterior rectal wall [68]. Palanivelu and colleagues [65] identified extrinsic compression during a flexible sigmoidoscopy in their patient with a large epidermoid cyst. A “bulging mass” was noted by Alavanja et al. [30] during a colonoscopy to work up a retrorectal lesion, ultimately determined to be a rectal duplication. Punctate mucosal erythema in association with a posterior fullness due to a retrorectal tumor was described by Abel and colleagues [85]. In the 20 % of duplications that communicate with the rectum, the connection may be demonstrated by endoscopy. Rarely, invasion of the rectum by the retrorectal tumor may be evident [1]. Glasgow et al. [50] found that proctoscopy alone was associated with a 52 % sensitivity for the diagnosis of a retrorectal tumor.
A plain radiograph of the pelvis is of limited value in the assessment of retrorectal tumors. Eighty-five percent of the plain abdominal roentograms were normal in the series from Grandjean and colleagues [52]. Wolpert et al. [9] suggest that positive findings, even sacral abnormalities, often are obscured by the superimposed bowel gas and surrounding soft tissue. The films may exhibit destruction or displacement of the sacrum or coccyx, a soft-tissue mass, or calcifications [9, 61]. An anterior sacral meningocele is affiliated with a pathognomic scimitar sign (a rounded, concave sacrum),absence of the coccyx, or both in 50 % of cases; this finding relates to abnormal bone development due to the mass effect from the tumor [59, 62]. Similar sacral anomalies are rarely seen with tailgut cysts. Hjermstad and Helwig [10, 20] encountered spina bifida occulta (n = 4), a healed coccyx fracture (n = 1), an anteflexed coccyx (n = 1), and an absent coccyx with a sacral bone deformity (n = 1) among their patients with a tailgut cyst; they commented that the sacrococcygeal abnormalities may arise from the same defect that produced the persistence of the postanal gut (i.e., tailgut cyst) [10]. Calcifications are primarily found in mature teratomas, although dermoid cysts may also demonstrate calcifications from tooth buds [27]. A calcification in a tailgut cyst may indicate a concurrent malignancy [20]. Sacral bone destruction is most reliably linked with malignancy [9, 45]. Localio and colleagues [43] noted that sacral bone destruction was displayed by 11 patients (92 %) with a malignant tumor, particularly in all eight cases of chordoma, but by none of the patients with a benign mass. Seventy-nine percent of the tumors with sacral bone destruction were malignant in the experience of Jao et al. [45




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








