Patient selection
BMI < 55
Compliant
Technical considerations
Tight sleeve (32 French)
Antral resection (2–3 cm from pylorus)
Avoid retained proximal fundus/thoracic migration
Follow-up
Reinforce strict dietary and exercise compliance
Target lower goal weight of BMI to allow for weight regain
Address weight loss plateaus and small regains early with nonsurgical intervention
Rates of Failure
Data showing the expected weight loss from sleeve gastrectomy is better reported than the causes of failure. In a recent systemic review of 123 papers including 12,129 patients undergoing SG or RYGB, those in the SG arm experienced an average excess weight loss of 66 % at 36 months [9]. This was comparable to those undergoing RYGB. Of the studies in this review who reported this data, 6.8 % (n = 118) of SG patients went on to have a subsequent bariatric procedure either for insufficient weight loss (n = 113, 95 %), reflux (n = 10, 8 %), or stricture (n = 5, 4 %). Many of the studies included patients who had their surgery early in the development of SG when surgeons utilized larger pouches and retained antrums. Despite this the failure rate remained low.
Long-term results of sleeve gastrectomy are also becoming better reported in the literature. Two recent papers show 50 % EWL persisting after 5 years, but that was after some weight regain from 3 to 5 years [10,11]. When weight regain occurs, it tends to be after 1 year and seems to be most prevalent between 3 and 6 years. Again, these results may represent early techniques with large pouches and heavier patients.
Anatomic Causes of Failure
The gastric sleeve created at the initial operation may not maintain its shape or orientation over the long term. This has been attributed to weight regain, inadequate weight loss, and the development of undesirable symptoms postoperatively. Antral dilation, retained fundus, and sleeve dilation are common findings in patients who fail initial SG (Fig. 26.1).
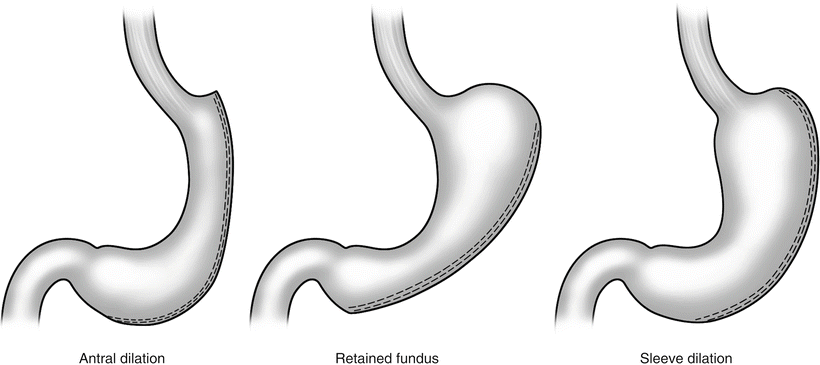

Fig. 26.1
Antral dilation, retained fundus, and sleeve dilation
In a review of SG failure, Weiner et al. reoperated on 106 patients who had initial sleeve gastrectomy [6]. Eighty-eight were reoperated on for insufficient weight loss (<50 % EWL) or weight regain, while 16 had severe GERD. More than 50 % of these patients had anatomic problems with their initial SG. In unpublished data presented at the 2011 American Society of Metabolic and Bariatric Surgery (ASMBS), the same author presented results of 1,434 patients evaluated after SG and found that pouch volume may increase from 56 to 186 cc (Weiner R, 2011, unpublished data). Antral dilation was the most common finding. These results are supported by published data of three-dimensional stomach volume analysis using computed tomography (CT) following SG, which showed average volume increase from 100 to 196 cc at 6 months post op [12]. Interestingly, in this limited series of patients, antral dilation did not always correlate with excess weight loss. Ten of the 27 patients in this study did have thoracic migration of the sleeve, and 4 of these developed persistent regurgitation after SG. Only 2 of the 17 patients without sleeve herniation complained of regurgitation. This supports the hypothesis that thoracic migration may contribute to GERD symptoms following SG. This may in turn lead patients to dietary habits that do not support weight loss in an attempt to control symptoms.
Likely the most contentious and best studied technical issue contributing to SG failure is that of the initial sleeve size—specifically, the size of the bougie used to calibrate the sleeve during its creation. Weiner et al. compared sleeves made without calibration to those made with 44 and 32 French bougies [6]. The un-calibrated sleeves had inadequate weight loss while that observed in those made with 32 French bougies was superior to the 44 French. Atkins et al. reported better excess body mass index loss (EBMIL) with 40 versus 50 French sleeves (60 ± 27.6 % and 45.4 ± 38.4 %, respectively) at 48 months (Fig. 26.2) [5]. Similar results were seen for resolution of dyslipidemia, diabetes mellitus, and hypertension. This was associated with a slightly higher leak rate in the tighter sleeves. Both groups had a 10 % weight regain by 4 years. A recent consensus statement from an international panel of experts recommends calibration with a 32–36 French bougie during SG [4]. They cite inadequate weight loss with larger sizes and increased complications with smaller.
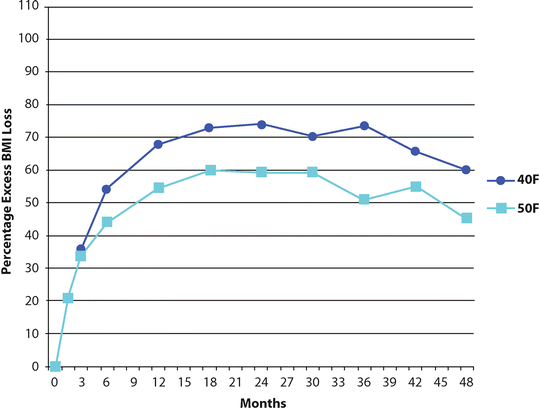

Fig. 26.2
Mean (± SD) percentage of body mass index (BMI) loss from baseline to 4 years after sleeve gastrectomy calibrated with either 40 or 50 French (F) bougies (Adapted from Atkins et al. [5])
Evaluating Failure
As previously described, the reasons patients fail to achieve the desired outcomes of their bariatric procedure are diverse. Hopefully, patients are complying with their diet and exercise programs and are being regularly evaluated for this during their scheduled follow-up. Early identification of failure and the symptoms and behaviors that may contribute to inadequate weight loss is important. Often minor, nonoperative interventions may be sufficient to get these patients back on track.
Once a patient has been identified as failing after a sleeve gastrectomy, we recommend a full history and physical exam. The history should focus on identifying symptoms of gastroesophageal reflux or obstruction. A review of the patient’s response to surgery so far including whether they achieved initial weight loss or resolution of comorbidities may lend clues to the cause of their failure. We recommend reviewing the food and exercise diary as well as reinforcing appropriate behaviors as the initial step in evaluating failing patients.
An upper gastrointestinal (UGI) contrast study should be performed to look for anatomical causes for failure. It is best to do the study yourself or at least be present for the examination to ensure that the appropriate concerns are addressed. The study should look for the presence and size of hiatal hernias, which could contribute to reflux symptoms and maladaptive eating. Segmental or generalized pouch dilations as well as the size of the antrum should be evaluated since these can limit the restrictive effect of the sleeve. The rate of pouch emptying should be rapid without delays in any segment or significant reflux into the esophagus.
The surgeon should perform an upper endoscopy. Signs of esophagitis, hiatal hernia, pouch dilation, and volvulus should be evaluated. This is also the best way to evaluate a chronic fistula. It is important to recognize that weight regain can occur without pouch dilation or anatomic abnormality. Also, stable weight loss may exist with a very malformed or dilated pouch. Thus, it is important to fully evaluate each patient prior to embarking on any intervention to address failure.
Managing Failure
To date, the options for managing inadequate weight loss, weight regain, or suboptimal resolution of the comorbidities following sleeve gastrectomy have been poorly studied and data is limited. No data exists on the nonoperative interventions in the failing patient. It is, however, intuitive that those patients who are not complying with dietary and exercise recommendations may be able to get back on track if they are willing to change these behaviors. Studies have shown that patients involved in close follow-up in comprehensive surgical weight loss programs have better results than those who are not [13–15]. It is important to ensure failing patients are compliant before offering them a surgical intervention. This is especially true since a noncompliant patient is unlikely to benefit from any surgical intervention and should not be exposed to the risks of another operation.
Several surgical options have been described to help failing patients lose further weight following sleeve gastrectomy. They can be divided into two groups: conversion to another type of bariatric procedure and revising the gastric sleeve to correct an anatomical complication.
Conversion to Another Bariatric Procedure
Since sleeve gastrectomy was originally described as the first stage of a duodenal switch procedure in super-obese patients, the option of converting a failing patient with an SG to a DS seems logical (Fig. 26.3). Dapri et al. compared doing a revision SG (n = 7) to DS (n = 19) in patients who failed initial SG [16]. Both groups had reasonable improvements in EWL (44 vs. 73 %, respectively); however, both immediate and late complications were much higher in the duodenal switch group. The published literature surrounding the DS procedure suggests that those patients undergoing a two-stage DS (SG followed by DS) had further reductions in EWL following the second stage [17]. Using this data as justification for conversion to duodenal switch in failing patients should be done with caution. Patients embarking on a planned two-stage DS in these studies were usually the super-obese (BMI > 60). They may not be reflective of patients failing SG performed with the intention of being the definitive procedure, but this option remains attractive for some.
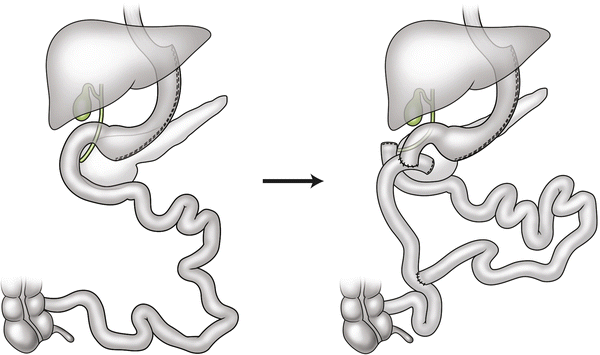

Fig. 26.3
Conversion of sleeve gastrectomy to duodenal switch
Another attractive solution in patients who fail to achieve the desired results after sleeve gastrectomy is to convert them to a Roux-en-Y gastric bypass (RYGB) (Fig. 26.4). This may also be effective at relieving undesirable symptoms from the sleeve including reflux, stricture, and obstruction. Unfortunately, it may also put the patient at higher risk of small bowel obstruction and ulceration—common complications of RYGB. Repeat surgery is also associated with a higher leak rate. Langer converted 11 % of 73 patients who underwent initial SG to RYGB with good results [18




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






