Indications
Failures
Weight regain
Technical compliance
Failure of weight loss
Metabolic
Recurrence of comorbidities
Complications
Time of onset
Acute (<7 days)
Early (7 days–6 weeks)
Late (6s–12 weeks)
Chronic (>1 week) (see Table 24.2)
Type
Surgical or anatomical
Nutritional or metabolic
Types of procedures
Revisions
Maintain basic anatomy of primary procedure
Conversions
Anatomy changed to different operation
Reversal
Restore original anatomy
Outcomes
Type of primary
Restrictive versus malabsorptive
Type of reoperation
See Table 24.3
Indications
Failures
Complications
Surgeon’s experience
Approach
Open
Laparoscopic
1.
Indications
2.
Procedure types
3.
Outcomes
Indications
In order to formulate a successful reoperative plan, it is paramount to understand the reasons why an additional procedure is needed. The main reasons for reoperation can be categorized as failures and complications.
Failure
Failure can be defined as inadequate weight loss or weight regain. Unfortunately, there is no consensus regarding the definition of failure after bariatric surgery. In general, “success” after bariatric surgery is defined as the durable control of weight loss with resolution or improvement of comorbid conditions and good quality of life. Several authors have utilized different parameters to assess success. The Adelaide study group proposed using an excess weight loss (EWL) >50 %, as previously described by Reinhold, whereas Fobi et al. utilize the term “failure” for EWL <40 % [5, 6]. To complicate matters even further, the parameters change for purely restrictive procedures laparoscopic adjustable gastric banding (LAGB), where failure is defined as <25 % EWL at 2 years [7]. It is clear, then, how failure of weight loss should be based on the expected average results of a particular operation, and not on a unified parameter.
Although inadequate weight loss might be a reflection of “failure,” it is certainly important to consider resolution or recurrence of comorbidities when evaluating patients for candidacy to reoperative surgery.
Failure is usually multifactorial and can be attributed to the surgeon (technical issues such as a large pouch in RYGB), to the patient (behavioral, dietary), and to the disease process itself (bariatric surgery “resistance”). It is then paramount to perform a thorough multidisciplinary preoperative evaluation to assess these key factors of failure (see section “Preoperative evaluation”).
In general, the majority of the reported second-line procedures are performed for failure of weight loss or because of weight regain [8].
Complications
Complications after bariatric surgery requiring reoperation can be classified based on time of onset: acute (7 days), early (7 days–6 weeks), late (6–12 weeks), and chronic (greater than 12 weeks). They can also be classified based on type: surgical or anatomical (hiatal hernia, marginal ulcer, slippage) versus nutritional or metabolic (malnutrition, nesidioblastosis, hypoglycemia) (Table 24.1).
The management of emergent complications (acute, early, and late), such as anastomotic leaks, bleeding, gastric remnant distention, anastomotic and bowel obstructions, and acute band prolapse are described in Part II of this book.
Late and chronic complications requiring elective reoperation vary based on the primary operation (Table 24.2). Some of the complications are present with different prevalences in most of the common bariatric procedures (i.e., malnutrition), whereas some others are specific to a particular procedure (i.e., gastro-gastric fistula, anastomotic stricture).
Table 24.2
Chronic complications of common bariatric operations
LAGB | VBG | RYGB | BPD-DS | JIB | Loop GB | LSG | |
|---|---|---|---|---|---|---|---|
G-G fistula | − | + | + | − | − | + | − |
Ulcer | + | + | + | − | − | + | − |
Anastomotic stricture | − | − | + | − | − | + | − |
Gastric outlet obstruction | + | + | + | + | − | + | + |
Foreign body erosion | + | + | +a | − | − | − | − |
Symptomatic GERD | + | + | + | + | − | + | + |
Bowel obstruction | +b | − | + | + | + | ± | − |
Malnutrition | +c | +c | + | + | + | + | + |
Procedure Types
Based on the degree of anatomic changes at the time of the reoperation, the types of procedures can be classified into the following: revisions, conversions, and reversals (Table 24.3).
Table 24.3
Types of reoperations
LAGB related | Revision | Band | Repositioning |
Replacement | |||
Port-tubing complex | Repositioning | ||
Reattaching | |||
Replacement | |||
Conversion | RYGB | ||
BPD-DS | |||
LSG | |||
Removal | |||
Non-LAGB related | Revision | RYGB | Pouch trimming |
Remnant gastrectomy | |||
Redo GJ | |||
Redo J-J | |||
Distal RYGB | |||
BPD-DS | Re-SG | ||
Limb shortening | |||
LSG | Re-SG | ||
Conversion | RYGB | BPD-DS | |
LSG | |||
VBG | RYGB | ||
BPD-DS | |||
LSG | |||
LSG | RYGB | ||
BPD-DS | |||
Loop GB | RYGB | ||
Reversal | RYGB | ||
BPD-DS | |||
VBG | Ring removal ± gastrogastrostomy | ||
JIB |
Revision
In revisional procedures, the modifications performed do not alter the basic anatomy of the primary procedure. Typically these procedures are reserved for chronic complications of an initially effective primary operation. Examples include gastric prolapse after banding procedures, gastric pouch and gastrojejunostomy revisions for recurrent ulcers, weight regain, and gastro-gastric fistulae.
Conversion
During the conversion, the structural anatomy of the primary operation is changed into a different type of operation. Most of the patients requiring these types of procedures present with failure of weight loss or weight regain [7]. However, lately with the increased number of laparoscopic sleeve gastrectomies (LSG) and late onset of gastroesophageal reflux disease (GERD) reported to be as high as 23 % at 5 years, many patients that had LSG need to be converted to RYGB or biliopancreatic diversion-duodenal switch (BPD-DS). Examples of conversion include modification from one restrictive procedure to another restrictive procedure or from a restrictive procedure to a malabsorptive procedure, and vice versa.
Reversal
The intent of the reversal procedure is to reestablish the original anatomy as closely as possible. Usual indications in this category include nutritional and metabolic complications (such as macronutrient and micronutrient malnutrition, liver failure) or patient noncompliance. Most of the bariatric operations can be reversed, unless they entail permanent removal of organs (such as LSG).
Outcomes
Most studies report higher perioperative morbidity (10–50%) and mortality (2.7–8.3 %) following reoperative procedures compared to primary ones [8–10]. Furthermore, when an additional procedure is necessary, the morbidity and mortality are even higher [10].
The primary endpoints of revisional surgery are safety (morbidity and mortality) and results (resolution of preoperative symptoms, comorbidity resolution, and weight loss). The outcomes differ based on the following criteria: indication for reoperation, type of primary operation, type of re-intervention, approach (laparoscopic versus open), and surgeon experience (Table 24.1).
Indications
In general, the success rate of a reoperation for a chronic complication of a primary operation (such as gastric outlet obstruction, marginal ulcer, gastro-gastric fistula, malnutrition, etc.) is superior to the success rate of re-intervention for failure of weight loss. This is largely due to the persistence of the noncompliant behaviors that led to failure of the original procedure.
In the chronic complication group, instead, the reoperation is largely successful in eliminating the anatomic derangement causing the preoperative symptoms.
Hence the appropriate patient selection can influence the overall outcome.
Type of Primary Procedure
The type of primary operation can also influence the weight loss of the secondary one. In fact, Brolin has shown how revisions of primary restrictive operations will result in higher weight loss than revisions of malabsorptive ones [11]. This could be explained by the superior initial benefit of the malabsorptive procedure not accounted toward the total weight loss after the revision. Instead, patients who failed the primary restrictive procedure never had significant weight loss, so most of the weight loss after the revisional malabsorptive operation is accounted for. A similar speculation can be applied to comorbidity resolution, which might have already occurred after the primary operation and not present at the time of reoperation. It is also important to mention how possible maladaptive eating behaviors that developed during the primary operation can be carried out after the re-intervention.
Type of Re-intervention
A wide variety of re-interventions are available (Table 24.3). Based on the frequency, these procedures can be divided into LAGB related and non-LAGB related. It is important to adopt this distinction in an effort to stratify the complexity of the procedure, which influences both the morbidity and the overall results.
Several reports have compared the outcomes of reoperative RYGB. Most of these reports consist of small to moderate size case series. In general, several studies have reported increased morbidity, mortality, and reoperation rates after reoperative procedures to RYGB [9]. In fact, re-interventions have a higher conversion from laparoscopic to open (up to 47.6 %), postoperative complications (up to 39.3 %), reoperation rates (up to 35 %), and mortality (up to 2.7 %) [9, 12].
More recently, Deylgat et al. have reported on 72 laparoscopic revisional RYGB without difference in the early and late complications rates and no mortality, suggesting safety of reoperative surgery [9]. Unfortunately, the authors provided no weight loss analysis. A recent study reviewed 121 patients who underwent open RYGB revision secondary to different primary restrictive operations (gastroplasties and both adjustable and nonadjustable gastric banding) [13]. The authors experienced a 3.3 % leak rate (significantly higher than the primary procedures), 8 % early reoperation rate, 10 % of symptomatic incisional hernias, similar 5-year weight loss results as the primary open RYGB, 93 % satisfaction rate, and no mortality.
Controversy exists in weight loss results after secondary operations. Only two case-matched studies of revisional RYGB have been published. Martin et al. reported on 27 open reoperative resectional RYGB [14]. He found significantly higher operating times, blood loss, and hospital stay in the reoperative group, but no differences in postoperative morbidity, resolution of comorbidities, mortality, and weight loss at 1 year. Zingg et al., in their analysis of 61 revisional RYGB matched with an equal number of primary RYGB, found that the rate of morbidity is significantly higher, and the rate of weight loss in the first 2 years is significantly lower in the revisional group (EWL 58.5 ± 34.9 % versus 85.9 ± 26.2 %) [12]. Possible reasons accounting for this difference have been attributed to several factors. The morbidity related to leaks after revisional surgery is likely related to the more fibrotic and possibly inflamed tissue encountered during the second operation. Furthermore, possible tissue ischemia can develop secondary to crossing of multiple staple lines, devascularization and trauma to the tissues, and inadvertent presence of staple lines too close to each other.
Other studies, instead, found no weight loss difference between primary and secondary operations, maybe because the maladaptive dietary and lifestyle behaviors persist after the reoperation [15].
The results of more complex reoperations (BPD-DS) are controversial as well. In fact, if some authors report safety of BPD-DS as a reoperative intervention, others weigh the efficacy against higher morbidity and mortality (62 and 8 %, respectively) [10].
Small studies directly comparing the standard revisional procedures, RYGB and BPD-DS, failed to demonstrate superiority of one over the other [16].
More recently several authors advocated the use of LSG as a second intervention. The potential benefits and the possible specific indications will be outlined later in the chapter.
Approach Open Versus Laparoscopic
Traditionally, most of the reoperations were approached with an open technique, especially when the primary operation was performed open. With the augmented experience, many centers have embraced the laparoscopic approach for reoperative bariatric cases, even in the face of multiple previous open operations.
Extrapolating the data from other comparisons between open and laparoscopic approaches, we can expect shorter hospital stay, shorter recovery times, and decreased postoperative pain for the laparoscopic procedures. Some authors have even proposed that the magnified view and the availability of finer instruments can offer an advantage in particular portions of the operation (such as the hiatal dissection and the pouch creation). This advantage could translate into a quantifiable difference in weight loss between laparoscopic and open primary procedures. Although one matched control study showed greater weight loss with the laparoscopic approach, a recent meta-analysis of six randomized controlled trials reported no difference between the two techniques [17]. The meta-analysis indicates a statistically significant shorter hospital stay, but higher reoperation rate and surgical time for the laparoscopic group [17]. However, no direct randomized comparison of laparoscopic versus open revisional surgery data is available at this time to draw a definitive conclusion.
Surgeon Experience
Similarly to other fields in surgery, surgeon experience has been well correlated to outcomes in bariatric surgery. A population-based study of the bariatric cases in the state of Pennsylvania has confirmed earlier findings of decreased perioperative mortality after bariatric surgery in the hands of experienced surgeons. In particular, surgeons who performed fewer than ten procedures per year had a twofold increase in 30-day mortality compared to surgeons performing more than 100 procedures per year [18]. Furthermore, the benefit of experience has also been extended to the hospital where the procedure takes place. In fact, the same authors, in recent analysis, reported how an experienced surgeon in a high-volume hospital has the lowest in-hospital and 30-day mortality (0.12 and 0.30 %, respectively), compared to the low-volume surgeon and hospital (0.57 and 0.98 %, respectively) [18].
Although no direct analysis has been published on reoperative surgery, we could apply similar concepts for the even more complex reoperations.
Preoperative Evaluation
In order to reduce the perioperative morbidity and mortality, as well as increase the success rate of reoperations, a comprehensive preoperative evaluation is necessary.
The primary reason for the comprehensive evaluation is to identify and correct the reason(s) for failure of the primary operation.
As previously mentioned, failure of bariatric surgery is often multifactorial. Besides the technical aspects of the primary procedure, the dietary and behavioral habits, along with intrinsic characteristics of the disease, play a key role in the overall success of the surgical intervention. Obviously the anatomical and functional causes of failure are more easily identified, whereas the behavioral ones are more resilient to intervention.
The preoperative evaluation should include the following elements: review of the primary operation technical details, obtaining recent anatomic and functional details of the gastrointestinal (GI) tract, preoperative dietary and nutritional assessment and counseling, psychological assessment, preoperative testing, and clearances.
Review of Operative Reports
Every bariatric operation presents a high degree of technical variability. In order to formulate a successful preoperative and intraoperative strategy, it is imperative to understand what was previously done and how. Often the patient understanding of what was done does not correlate to reality. It is then necessary to review the primary procedure operative report in order to understand the type of procedure performed and the technical details. If the patient has a LAGB implanted, important details include the type of band, the technique utilized (perigastric versus pars flaccida), type of port, and location. For the gastric bypass, important technical details include pouch size, type (RY, loop, divided, nondivided), presence of foreign bodies (anastomotic rings, bands), limb lengths, limb route, and previous use of gastrostomy tubes.
Imaging and Functional Studies
In spite of knowing the type of primary operation performed, several key changes can occur over time—hence, the need for up-to-date anatomic and functional assessment of the GI tract prior to each reoperation.
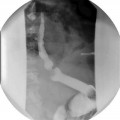
A preoperative contrast upper GI (UGI) study is the best radiologic examination to obtain an anatomic road map. The test will also provide a functional assessment of the GI tract visualizing potential delay of transit at stricture sites, abnormal dilatations, and fistulous communications. It is important for the operating surgeon to personally review the images in order to have a clear understanding of the study results. Specific diagnostic suspicions should be communicated to the radiologist in order to obtain the best possible study. In fact, the diagnostic dilemma can influence the technical aspects of the study and the radiologist might elect to obtain specific views (supine, decubitus, etc.) in order to clarify the scenario.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree