Fig. 19.1
(a) Upper GI contrast study demonstrating a leak from the proximal staple line following sleeve gastrectomy. (b) Computed tomography (CT) scan of the abdomen showing the staple line of the sleeve gastrectomy with contrast extravasation proximally into an extraluminal collection (arrow) immediately adjacent to the gastric sleeve staple line
In 2009, the American Society for Metabolic and Weight Loss Surgery (ASMBS) published a position statement on prevention and detection of gastrointestinal leak after gastric bypass including the role of imaging and surgical exploration [8]. The sensitivity of upper GI contrast study varies among reports between 22 % and 75 %. When upper GI and CT are combined, up to one-third of patients with leaks will have both studies interpreted as normal. Therefore, the position statement suggested that laparoscopic or open reexploration should be an appropriate diagnostic option when gastrointestinal leak is suspected, as reliance on false-negative imaging studies may delay operative intervention.
Management
Management of leaks after bariatric surgery varies and depends on the extent of the disruption, the extent of abdominal contamination, the site of the leaks, and timing of presentation. The initial treatments should include NPO (nothing per orem), early initiation of broad-spectrum antibiotics, and fluid resuscitation. Leaks can be classified as acute (within 7 days), early (1–6 weeks), late (6–12 weeks), and chronic (>12 weeks) [9]. The treatment options for postoperative leaks after bariatric surgery depend on the timing of leaks at presentation. Management of early and acute leaks includes conservative nonoperative management, reoperation with abdominal washout, closure of the defect or placement of a T-tube to intubate the defect, and wide peritoneal drainage. Alternatively, there have been few reports on the use of intraluminal stenting for gastric leaks after Roux-en-Y gastric bypass and sleeve gastrectomy [10–15]. For late and chronic leaks, management may require performing a proximal gastrectomy with esophagojejunostomy [16]. These management strategies are depicted in Fig. 19.2.
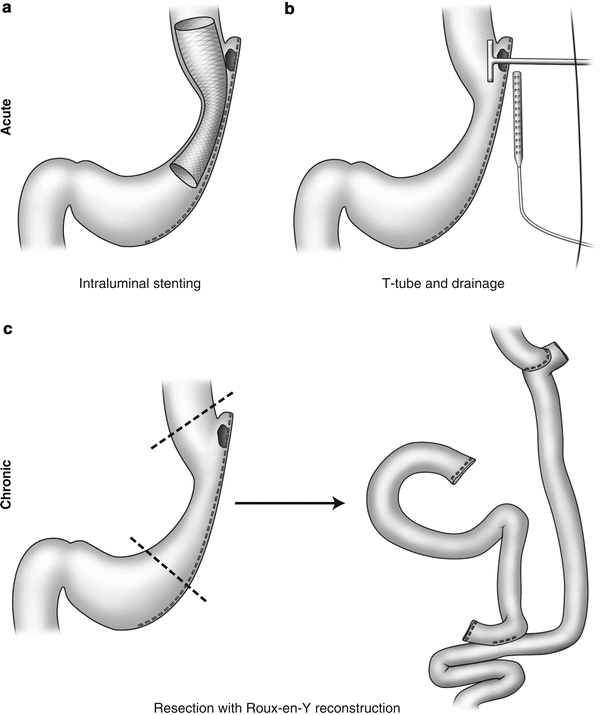

Fig. 19.2
Management strategies for staple-line leak. Acute presentations can often be managed successfully with (a) insertion of intraluminal stent to cover the staple-line defect or (b) drainage and T-tube insertion. (c) Chronic leaks from the staple line occasionally warrant resection of the involved area with Roux-en-Y reconstruction
Nonoperative Treatment
Nonoperative treatment can be considered for small, contained leaks, particularly in hemodynamically stable patients without peritoneal signs on abdominal exam. The patient should be kept NPO (nothing per oral) with total parenteral nutrition, and broad-spectrum intravenous antibiotic should be administered. The course for nonoperative treatment should be followed with measurement of serial white blood cell (WBC) count, vitals, and physical exam. Patients with increasing WBC, persistent tachycardia, or worsening of abdominal pain should proceed to operative management. In a relatively large study of leaks after gastric bypass, Gonzalez and colleagues reported successful nonoperative treatment in 23 of 26 patients, with an overall morbidity of 62 % and no mortality [17]. Similarly, Casella et al. reported successful nonsurgical treatment of staple-line leaks after laparoscopic sleeve gastrectomy in six patients to include percutaneous drainage along or percutaneous drainage with endoscopic stenting or injection of fibrin glue [18].
Reoperation and Drainage
The mainstay of surgical treatment includes drainage of all fluid collections and placement of abdominal drains. Additionally, some surgeons make an attempt at closure of the defect; however, these closures tend to break down due to poor tissue integrity at the leak site. Leaks at the jejunojejunostomy or the gastric remnant may be more amenable for primary closure, and revision of the anastomosis is rarely needed [2]. An alternative approach to control the leak site is placement of a T-tube directly into the defect [14]. This technique consists of obtaining a conventional T-tube drain and placing the T part of the drain directly into the defect (Fig. 19.2). The drain is then exteriorized and placed to bag drainage. The T-tube is left in place for 4–6 weeks and is slowly withdrawn over time (1–2 in. per week.). The idea here is to create a track along the drain, hence creating a controlled fistula. Upon withdrawal of thetube, the well-formed fistulous track will collapse and eventually close.
Endoscopic Stent
Endoscopic stenting for management of bariatric leaks is a relatively new concept and was initiated from the experience of using endoscopic stenting in management of esophageal anastomotic leaks after esophagectomy [19]. It is important to note that the use of endoscopic esophageal stent for management of leaks is an off-label use of a US Food and Drug Administration (FDA)-approved device for malignant esophageal strictures or fistulas. Table 19.1 demonstrates selected series describing the use of endoscopic stent for treatment of gastric bypass or gastric sleeve leaks (Fig. 19.3) [10–15, 20]. Serra and colleagues reported on the use of coated self-expanding stents for management of leaks after sleeve gastrectomy or duodenal switch in six patients with control of leaks in 83 % of cases [11]. Casella and colleagues reported the use of endoscopic stent for leak at the gastroesophageal junction after sleeve gastrectomy in three patients, with complete healing occurring in all patients [18]. Oshiro and colleagues reported successful management of proximal gastric leak using a covered endoscopic stent in two patients who underwent prior unsuccessful laparoscopic treatment for the leak [13]. In contrast, the largest series of eight cases of endoscopic stent for leak after sleeve gastrectomy was reported by Tan and colleagues [14]. They reported a 50 % success rate for closure of the leak, with four patients requiring premature removal of the stent due to either migration, hematemesis, or obstruction from kinking at the proximal aspect of the stent. One of the major difficulties with usage of stents for control of leaks is their ability to migrate. Eubanks and colleagues described the use of endoscopic stenting in 19 patients, although they described an overall healing rate of 84 %, stents had to be repositioned or replaced in 47 % of patients due to migration. Three patients required reoperation for stent removal. In addition, endoscopic stenting was noted to be less effective for management of chronic fistula in this series. In another report, Fukumoto and colleagues reported a single case of endoscopic stent for leak after sleeve gastrectomy without success that required operative closure of the fistula [15].
Table 19.1
Outcomes in management of leaks after selected series of gastric bypass and sleeve gastrectomy
Authors/year/[reference] | Procedure (no. of patients with leaks) | Incidence of leaks (%) | Treatments | Resolution of leaks following stent insertion (%) | Overall mortality |
|---|---|---|---|---|---|
Sakran N. 2013 [15] | Sleeve | 44 | Reop (n = 27) | Endoscopic stent with laparoscopic drainage | 9.1 % |
Perc drain (n = 28) | |||||
Stent (n = 11) | |||||
De Aretxabala X. 2011 [21] | Sleeve (n = 9) | – | Lap reop (n = 5) | 75 % | 0 % |
Perc drain (n = 1) | |||||
Open reop (n = 3) | |||||
Stent (n = 4) | |||||
Csendes A. 2010 [22] | Sleeve (n = 16) | 4.7 % | Nonoperative (n = 8) | – | 0 % |
Reop (n = 8) | |||||
Tan JT. 2010 [14] | Sleeve (n = 14) | – | Nonoperative (n = 4) | 50 % | 0 % |
Reop (n = 7) | |||||
Stent (n = 8) | |||||
Casella G. 2009 [18] | Sleeve (n = 6) | 3.0 % | Perc drain (n = 5) | 100 % | 0 % |
Stent (n = 3) | |||||
Freedman J. 2012 [23] | Bypass (n = 69/2214) | 3.1 % | Nonoperative (n = 6) | 100 % | 0 % |
Stent (n = 35) | |||||
Reoperation (n = 28) | |||||
Thodiyil PA. 2008 [24] | Bypass (n = 46) | 1.7 % | Reop (n = 27) | – | 0 % |
Conservative (n = 33) | |||||
Ballesta C. 2008 [25] | Bypass (n = 59) | 4.9 % | Reop (n = 23) | – | 8.5 % |
Conservative (n = 36) |







