Fig. 48.1
(a) Gastrografin enema shows residual sigmoid colon and diverticula. (b) Gastrografin enema shows a leak at the top of the Hartmann’s pouch with extravasation and intraluminal contrast into the small bowel
Intraoperative Considerations
Patient Positioning
Anticipate a long procedure and pad the patient’s bony prominences accordingly. The patient may be placed in the lithotomy position in Lloyd Davies or Allen Yellofin® stirrups (Allen Medical Systems, Acton, MA). Care should be taken to avoid pressure on the peroneal nerves and the hips. The overall position is aimed to maintain symmetric hip extension, knee flexion, and thigh abduction. Extreme hip extension beyond 60° occasionally can lead to femoral nerve palsies if a self-retaining retractor is positioned against the extended extremity. The perineum should be slightly over the table to ensure easy passage of the EEA stapler. Rectal washout can be performed and a mushroom catheter left in the rectum if desired to facilitate identification of the Hartmann pouch. A proctoscope and or sizer may also be used intraoperatively to identify the pouch. The vagina should also be included in the preparation. Alternatively, our preference is to position the patient supine on a split leg table. The split leg table avoids potential difficulties with a longstanding lithotomy position, including nerve injuries and compartment syndromes. Once again, care must be taken to ensure that the patient is positioned far enough down the table so that access to the anus (to pass the EEA stapler) can be readily achieved. Use of a beanag with the patient’s arms tucked at the sides can be helpful to ensure the patient does not slip cephalad on the table, especially when in the steep Trendelenburg position.
Approach to the Procedure
The procedure may be undertaken using a laparoscopic or an open approach. Adhesions encountered from previous surgery or prior infection may make a laparoscopic approach impossible. The extent and degree of adhesions may be difficult to predict when, on occasion, less severe adhesions are encountered than anticipated and the procedure progresses quite smoothly. Alternatively, with extensive adhesions, bowel injury may occur when attempting to enter the peritoneal cavity. A reasonable approach is the use of a “Peek port,” which entails entering the abdomen through a small incision and assessing the degree of adhesions [14]. Additional disposable laparoscopic equipment such as trocars is not opened until the feasibility of a laparoscopic approach is determined. Alternatively, a port also can be placed away from the site of the previous surgery to assess the degree of adhesions and the feasibility of a straight laparoscopic approach.
Exposure and Lighting
The importance of having adequate exposure and lighting during reoperative surgery cannot be overestimated. If an open approach is used, the incision should extend to the symphysis pubis. Cephalad extension of the midline incision may be needed if mobilization of the splenic flexure is needed. Operating between the patient’s legs provides optimal visualization of the splenic flexure, as does rotation of the table to a left-side-up position.
Adequate lighting in the operating room, a headlight, lighted pelvic retractors, or all of these are helpful. A self-retaining retractor with a bladder blade is used. Straight blade and curved (Deaver) retractors are available; the former are more helpful for deep pelvic dissection, which is, on occasion, necessary to free up the Hartmann’s stump. Care must be taken to avoid placing these retractors on the drapes because of a risk of flammability.
Initial Dissection
The initial dissection is focused on lysing all small-bowel adhesions in the pelvis to be able to identify the Hartmann’s pouch. Ultimately, in the majority of cases, all small-bowel adhesions from the ligament of Treitz to the ileocecal valve are lysed to enable mobilization of the colostomy and bring the proximal colon down to the pelvis without tension. The pelvic dissection associated with a prior Hartmann’s resection may be challenging secondary to dense adhesions and the inability to distinguish a plane suitable for dissection. It is advisable to lyse the filmy small-bowel adhesions first and attack the more difficult adhesions later. Dense adhesions often occur at the top of the Hartmann’s pouch, and encountering staple material is an indication of proximity to this structure. If extremely dense adhesions are encountered, hydrodissection, or infiltration of the fused area with saline with a small-gauge needle, may be helpful [15]. The appendix also can be drawn down into the pelvis toward the Hartmann’s stump and may occasionally lead the surgeon to believe he or she has encountered the right ureter. The ureters may also have a more medial position after prior surgery, and the left ovary and tube in particular may be fused with the top of the Hartmann’s pouch. Bleeding from the pelvic side wall may often occur from entering the fallopian tubes or a branch of the ovarian vessels.
The colostomy is mobilized, incising the mucocutaneous junction and trying to preserve all the mesenteric attachments. Injection with saline around the mucocutaneous junction circumferentially may facilitate dissection. The stoma is resected and fresh bowel used for the intended anastomosis. Once the stoma is mobilized, the surgeon generally can assess whether there is adequate length for a tension-free anastomosis. Additional length is facilitated by a number of maneuvers including division of the lateral colonic attachments, takedown of the splenic flexure, division of the inferior mesenteric artery at the takeoff of the aorta, and division of the inferior mesenteric vein at the inferior border of the pancreas. Alternatively, further length can be achieved by mobilizing the rectum further and essentially bringing the Hartmann’s pouch up to the proximal bowel. Once complete mobilization of the proximal colon is performed and adhesiolysis is completed, the small bowel and colon can be packed into the upper abdomen.
Identification and Mobilization of the Hartmann’s Pouch
Once the small bowel is mobilized, the top of the Hartmann’s pouch can be identified. Some surgeons mark the top of the pouch with a long nonabsorbable suture to facilitate identification. We generally have not found this to be helpful. The staple line of the Hartmann’s pouch is identified where the length of the pouch is usually longer than anticipated, even if it is located below the pelvic brim. If the staple line is adherent to the presacral fascia, it is generally safe to commence the dissection in the midline posteriorly, thus avoiding the ureters and the iliac vessels. It is not uncommon for the superior rectal artery to be left intact, and placing a Babcock clamp on the end of the Hartmann pouch and applying cephalad traction facilitates identification of the mesentery and straightening of the rectum. It is our practice to mobilize and dissect out the Hartmann’s pouch at least from the mid- to the proximal rectum. This is generally necessary in an effort to “straighten out the rectum,” which often has a concertina-like configuration. Once the Hartmann’s pouch is mobilized, a small sizer is placed per rectum to ensure that this passes easily to the area of the intended anastomosis. In those patients who have had significant sepsis or in those who have had a longstanding Hartmann’s pouch, further mobilization may be needed. We have found that in women, further dissection is often required in the anterior cul-de-sac because the mid-rectum tends to angulate and adhere to the uterus. Despite further mobilization, some patients may still have a fairly fibrotic pelvis in which the rectum is intrinsically normal but the surrounding tissues fibrotic enough that it is impossible to pass a sizer; in this case, an EEA stapled anastomosis may not be feasible and a hand-sewn anastomosis is preferable. The top of the intended site of the anastomosis is then re-resected and the integrity of the rectum tested by filling the pelvis with saline and insufflating the Hartmann’s pouch.
The ureters should be identified and the surgeon should be aware that they may be in an unanticipated position, particularly drawn in more medially, after prior surgery. Ureteral stents may be used in selected cases with prior severe pelvic sepsis or unclear anatomy. Stents do not prevent ureteral injury but facilitate the recognition of such injury. We do not routinely use ureteral stents for Hartmann’s reversal. The vagina may be adherent to the rectum, and its dissection can be facilitated by placing a finger in the vagina, which may assist in the dissection.
Performing the Anastomosis
We prefer using an EEA stapler to perform the anastomosis after Hartmann’s resection. The anvil is placed in the proximal bowel. A hand-sewn purse string is placed or a purse string device may be used. The small then larger sizer is introduced through the rectum and gently guided to the toop of the Hartmann’s pouch. Occasionally it is difficult to introduce the stapler into the anus, and Khoury and Opelka [16] have reported placement of a Faensler or Chelsey-Eaton anoscope with gradual dilatation of the sphincter and placement of the stapler shaft through the anoscope. The EEA stapler is guided through; the trochar exits at the top of the Hartmann’s pouch, where the anvil is snugged up and secured, and the stapler then is fired. The instrument generally is removed easily and the tissue rings inspected for thickness and integrity. The anastomosis then is tested by occluding the bowel proximally and introducing air through a proctoscope or through a flexible sigmoidoscope [17].
Alternatives
There is no one single technique to perform an anastomosis after a Hartmann’s takedown and some ingenuity employing other techniques may be necessary. The stapler may not pass up to the top of the rectum because of fibrosis and contraction, particularly if the patient has been diverted for many years. In this case, there are several alternatives. One option is to perform a hand-sewn anastomosis. Another option is to introduce the stapler and bring the trochar through the anterior rectal wall, thus performing an end of colon to side of rectum anastomosis (Fig. 48.2) [15]. Further options include employing a double purse-string technique in which a purse string is placed in the proximal colon and the distal end (rectum). The stapler still is introduced through the anus. A final technique employs a single purse string in the rectum and placing the stapler through the side of the proximal colon and completing the anastomosis by transecting the end of the colon with a TA stapler.
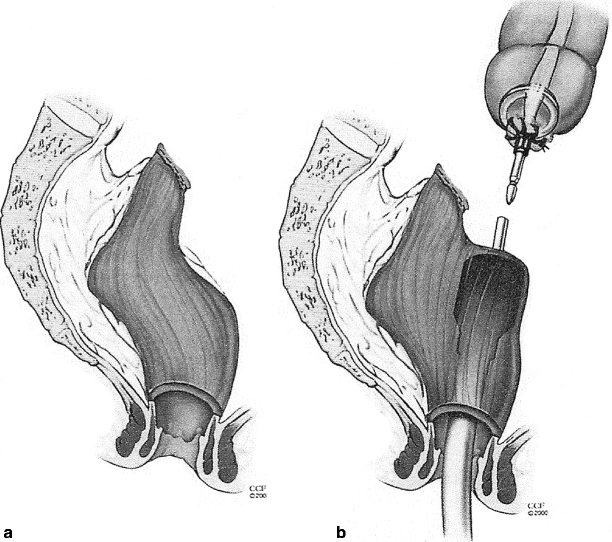
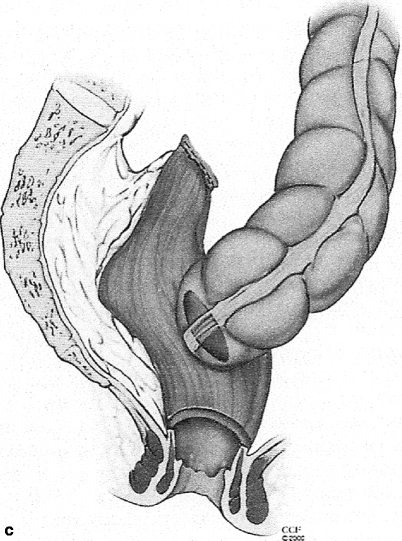


Fig. 48.2
Mobilization of the Hartmann’s stump may be difficult, especially if the apex is scarred (a); in this case the EEA stapler may be guided through the anterior wall of the rectum (b) for intended end of colon to side of rectum (c) anastomosis (© CCF 2000, reprinted with permission from the Cleveland Clinic Foundation)
Abdominal Wall Closure
After completion of the anastomosis, the abdomen is irrigated and the incision closed. A mass closure technique is superior to layered closure. A continuous abdominal wall closure is associated with a lower risk of abdominal wound dehiscence. Furthermore, a meta-analysis of six randomized, controlled trials found that the risk of incisional hernia formation was significantly less with a continuous compared with an interrupted closure (regardless of the suture type used) [18]. The use of resorbable versus nonresorbable sutures results in no difference in dehiscence rates, but nonresorbable sutures result in higher rates of persistent sinus formation and chronic wound problems. Optimal primary wound closure is with a mass closure continuous technique with resorbable sutures placed at an interval of 1 cm apart and 1 cm back on the fascia [19]. Laparoscopic techniques minimize incision length and wound trauma and may be associated with fewer wound complications.
Reoperation for Sepsis and Anastomotic Complications after a Hartmann’s Takedown
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








