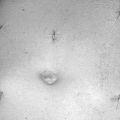
Fig. 28.1
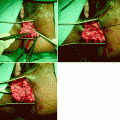
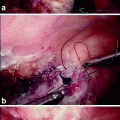
Defect of the rectovaginal septum as seen during intraoperative dissection in a patient with rectocele and symptoms of obstructed defecation. The rectovaginal septum is grasped with forceps, exposing the cranial defect (arrow)
Etiology
There is little chance of making any headway in the investigation of the etiology of rectocele and rectoenterocele unless there is agreement on what this term actually signifies. If a rectocele is a diverticulum of the rectal ampulla that protrudes into the vagina, then there must be some form of structural failure for this to occur. Put another way: in a normal person there needs to be some form of barrier that preserves the pressure differential between the lowermost aspect of the rectal ampulla (intra-abdominal pressure) and the vagina, which is at atmospheric pressure below the vaginal high-pressure zone defined by the puborectalis muscle [7]. It is hard to see how this pressure differential could be maintained without a strong fascial barrier, and the rectovaginal septum or “Denonvilliers’ fascia” would seem to represent that barrier. Because a defect of the rectovaginal septum gives rise to a rectocele, it is best seen as a problem that primarily is not of the rectum itself, but of the rectovaginal septum [6].
Although it seems possible to view the rectovaginal septum on ultrasound imaging [8, 9], this requires endoluminal transducers (Fig. 28.2), which interfere with functional assessment, and most rectoceles and enteroceles are only apparent once structures come under load (e.g., during a Valsalva maneuver). Defects of the rectovaginal septum, however, can be visualized on cadaveric dissection [10, 11] and intraoperatively using a vaginal approach (see Fig. 28.1). Dissection requires great care, and during posterior longitudinal colpotomy the septum is often divided with the vaginal wall [4] and hence overlooked. This may explain why its existence and role often are not appreciated.
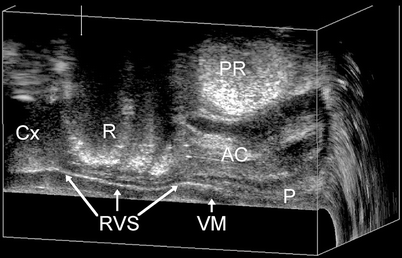

Fig. 28.2
The rectovaginal septum (RVS; Denonvilliers’ fascia) as seen on static endovaginal three-dimensional ultrasound at rest. A hyperechogenic sheet is seen to extend from the perineal body (P) to the cervix (Cx), separating the vaginal muscularis (VM) from the rectal ampulla (R) and the anal canal (AC) puborectalis muscle PR
Research into the etiology of rectocele, therefore, requires specialized imaging. It has been possible to demonstrate a true rectocele in about 10% of young, nulliparous women [12], and this finding is associated with body mass index. Childbirth seems to have some effect on the prevalence of rectocele [13], and preexisting rectoceles seem to be enlarged by vaginal childbirth. The size of the levator hiatus and trauma to the levator ani muscle, however, do not seem to have any major impact on rectocele appearance [14, 15].
Chronic constipation, chronically increased intra-abdominal pressure, and obesity are all supposed to promote rectocele, and constitutional factors such as a deep cul-de-sac also have been implicated [6].
An enterocele adds another layer of complexity to this already rather complex issue. It seems clear that hysterectomy is a risk factor, as is a congenitally deep cul-de-sac [16]. Occasionally, an enterocele seems to be congenital, and this is probably associated with the size of the levator hiatus [16], much more so than in the case of a rectocele. However, it also should be clear that an intact rectovaginal septum ought to prevent protrusion of enterocele into the vagina. This implies that the most important component of any enterocele repair may well be reconstruction of the rectovaginal septum. Finally, the role of the perineal body in the etiopathogenesis of these conditions currently is poorly defined. For many decades, pelvic reconstructive surgeons have claimed that a successful prolapse repair requires a perineoplasty, but there are no trials examining this assumption.
Diagnosis
For gynecologists, the diagnosis of a rectocele and a rectoenterocele is almost exclusively clinical, although the distinction between the two is often achieved only intraoperatively, and then rather poorly. In general, descent of the posterior vaginal wall (e.g., as quantified by the prolapse quantification system of the International Continence Society) [17] is taken to imply a rectocele without a perceived need for further diagnostic measures [6]. This is, I believe, a grave misunderstanding. Clinically, posterior compartment descent is diagnosed as a “rectocele,” ignoring that at least five different conditions can lead to apparent or actual prolapse of the posterior vaginal wall. A second-degree rectocele could be due to a true rectocele (i.e., a defect of the rectovaginal septum), which is the most common feature and which is associated with symptoms of prolapse, incomplete bowel emptying, and straining during evacuation [18]. Alternatively, it may be due to an abnormally distensible but intact rectovaginal septum, which is most commonly associated only with prolapse symptoms. Another scenario is as a consequence of a combined rectoenterocele, which is less common, an isolated enterocele (uncommon), or just secondary to a deficient perineum, giving the impression of a “bulge” [19]. Occasionally, a “rectocele” turns out to be due to rectal/rectoanal intussusception as an early stage of rectal prolapse: the wall of the rectum is inverted and enters the anal canal during a Valsalva maneuver. It may be possible to diagnose true rectocele and even intussusception by rectal examination during straining, but this does not seem ever to have been formally assessed. Clearly, clinical examination is insufficient for the assessment of rectocele [20].
For colorectal surgeons and radiologists, defecation proctography (DP) has been regarded for about two decades as the gold standard investigation in women with suspected rectocele, and the use of this technology has allowed substantial progress in the management of this condition [6]. However, correlations between DP findings, clinical findings, and symptoms are poor [21], and this has slowed down and narrowed the uptake of this technology. Lately, dynamic magnetic resonance imaging and magnetic resonance defecography have assumed greater prominence despite the substantial cost, inconvenience, and lack of availability of this method for functional gastrointestinal use. It is debatable whether supine forced/simulated defecation in a magnetic resonance tube replicates the physiological process of defecation, and even seated defecation in front of a fluoroscopy system or open-architecture magnetic resonance system proctography may be criticized on that point. Another diagnostic approach has been ultrasonography, either translabially [22] or endovaginally [23]. In general, ultrasound is performed with a maximal Valsalva maneuver mimicking defecation without any form of patient preparation, which further simplifies the diagnostic procedure and reduces both cost and patient embarrassment [19]. This technique was first described by gynecologists using linear and curved-array transducers normally employed for abdominal imaging [24, 25]. Colorectal researchers have described this technique transperineally using intrarectal (and intravaginal) ultrasound gel for contrast [26, 27], although stool usually provides sufficient contrast between the rectal lumen and the vagina.
It is not surprising that this approach is greatly preferred by patients [28], and the results in terms of diagnosis and rectocele size seem to be largely equivalent to those obtained by DP [28, 29]. The main advantage of translabial ultrasound is the ease with which a diagnostic work-up can be obtained while also assessing the other vaginal compartments, including the urethra and the remainder of the pelvic floor [30]. This is particularly useful in view of the fact that many women with defecatory disorders suffer from other forms of pelvic floor dysfunction, necessitating a multicompartment approach is necessary [31].
In urogynecological units, patients with rectocele and rectoenterocele frequently present with urinary incontinence and prolapse of other compartments. Posterior compartment prolapse is a highly prevalent, long-term complication of colposuspension procedures [32, 33]. It is common for rectoceles to be asymptomatic and it is possible that poor stool quality may contribute to obstructive symptoms [34]. However, rectocele clearly is associated with symptoms of a vaginal lump and those of obstructed defecation, such as incomplete emptying, straining during evacuation, and the need for vaginal digitation and perineal manipulation to assist defecation [18, 34]. Gynecologists need to be prepared to consider and evaluate symptoms of obstructed defecation, and colorectal surgeons should not disregard the symptoms of vaginal prolapse and bladder dysfunction. Perhaps more than any other fields, those two specialties must communicate and cooperate in cases of pelvic floor dysfunction[31].
Rectocele
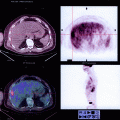
An anterior rectocele is evident on translabial ultrasound as a diverticulum of the anterior wall of the rectal ampulla into the vagina and generally is apparent only during the Valsalva maneuver (Fig. 28.3) and not at rest. Figure 28.4 shows a comparison of fluoroscopy and translabial ultrasound in a patient with a typical rectocele, contrasting appearances. Posterior rectoceles are uncommon in adult women and may be a form of intussusception rather than an actual rectocele (Fig. 28.5). A rectocele usually contains iso- to hyperechoic feces and often there is bowel gas as well, resulting in specular echoes and reverberations. Occasionally, there is no stool in the ampulla that could be propelled into the rectocele, and as a result it remains smaller and filled only with rectal mucosa. Because distension of a rectocele will depend on the presence and quality of stool, appearances may vary considerably from one day to the next. The severity of a rectocele can be quantified by measuring maximal descent relative to the inferior symphyseal margin and by determining the maximal depth of the sacculation (as seen in Fig. 28.4, which compares ultrasound and radiological findings in a patient with rectocele).
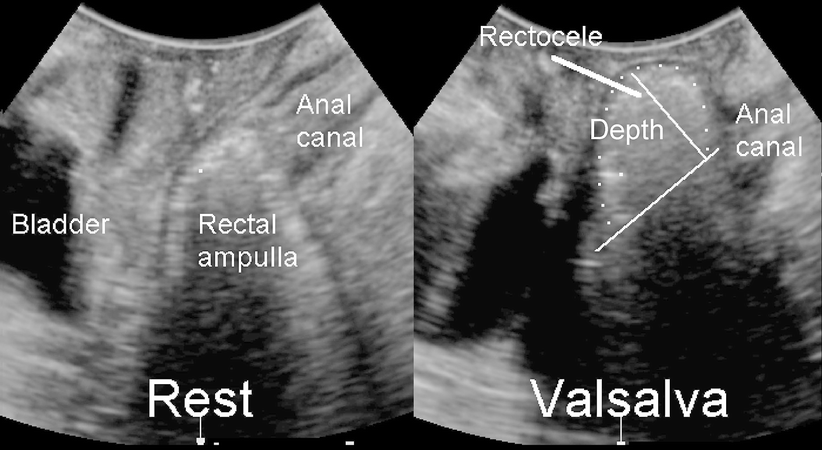
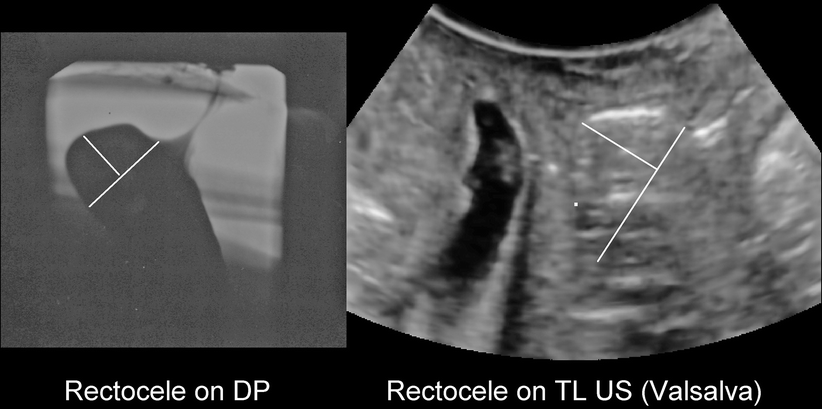
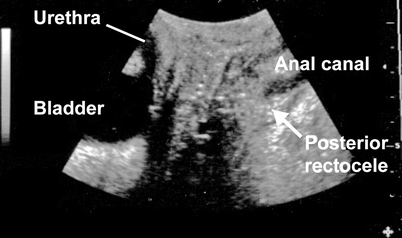

Fig. 28.3
Translabial imaging of the posterior compartment at rest (left) and during Valsalva. The two perpendicular lines illustrate how rectocele depth is measured: a line is placed extending the contour of the anal canal, and depth is measured vertical to that first line. The rectocele is outlined by white dots

Fig. 28.4
A typical true rectocele as seen on defecation proctogram (DP; left) and open translabial ultrasound (TL US; right). Whether such a rectocele is symptomatic will, to a large extent, depend on stool quality, and many are asymptomatic (Reprinted with permission from Perniola et al. [28]). The translabial ultrasound als shows substantial bladder neck mobility and a 3rd degree cystocele to the left of the rectocele

Fig. 28.5
Posterior rectocele (arrow) in patient with symptoms of obstructed defecation. This appearance is uncommon in adults
Figures 28.6 and 28.7 demonstrate the appearances on three-dimensional and cross-sectional (tomographic or multislice) ultrasound, showing clearly that rectoceles commonly are symmetrical and located at the inferior margin of the rectal ampulla. From an imaging point of view there are no “low” or “high” rectoceles, as have been previously described.
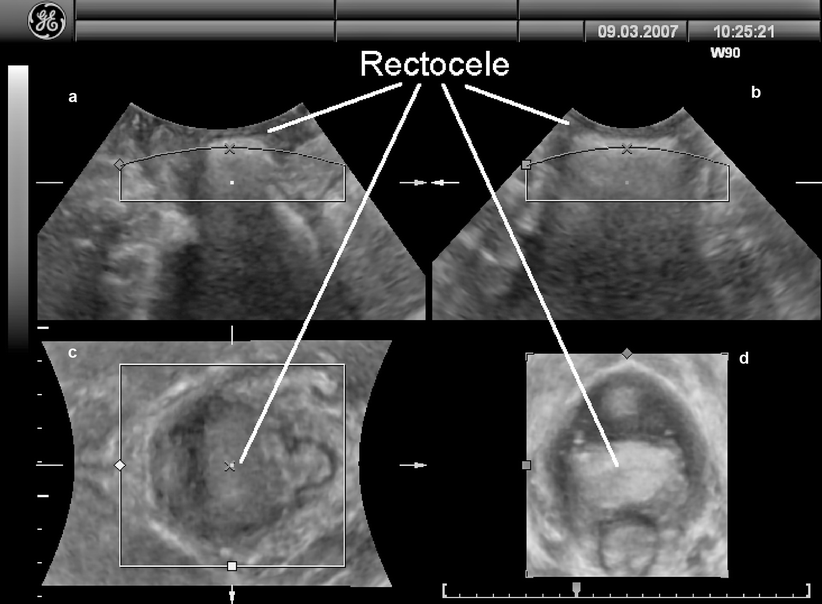
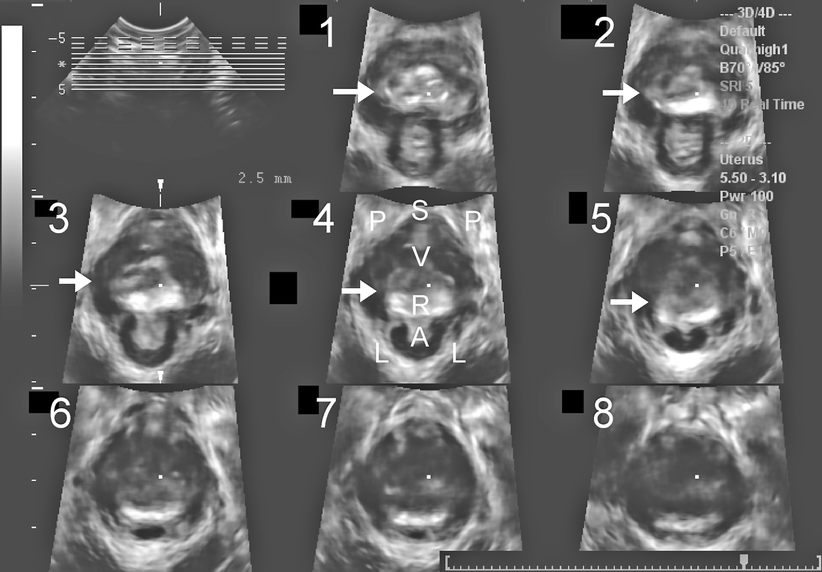

Fig. 28.6
A typical rectocele as seem on three-dimensional translabial ultrasound, showing that the ampullary diverticulum is completely symmetrical. (a) shows the standard midsagittal view, (b) a coronal view, (c) is the axial plane at the level of the levator hiatus, and (d) represents the same in a rendered volume

Fig. 28.7
Tomographic imaging of a typical rectocele (R), showing its symmetry and extent, illustrating how it fills the entire levator hiatus. Tomographic ultrasound is obtained from volume data acquired during maximal Valsalva. Slices are arranged from 5 mm below (1) to 12.5 mm above (8) the plane of minimal hiatal dimensions [55], which is represented in slice 3. Arrows indicate the rectocele. S symphysis pubis, P pubic rami, V vagina, R rectocele, A anal canal, L levator ani
Enterocele
An enterocele is visualized as a downward displacement of abdominal contents into the vagina ventral to the anal canal. This finding is highly sensitive to bladder and bowel filling, and this is one of the main reasons why prolapse assessment (on imaging or clinically) should be undertaken after voiding and, if possible, defecation. At times a repeat assessment may be indicated if the patient is unable to evacuate a full rectum. Small bowel may be identifiable by its peristalsis and sometimes by intraperitoneal fluid outlining the apex of the enterocele. Distal shadowing is much less common than with rectocele, and often the contents have an irregular isoechoic or ground-grass-like appearance (Fig. 28.8). A sigmoid enterocele (sigmoidocele) tends to show coarser ultrasonographic patterns, as seen in Fig. 28.9, and may even appear similar to rectal contents alone. A special case is rectal intussusception, which appears as an enterocele that inverts the anterior wall of the rectal ampulla into the anal canal, resulting in a trumpet-like distension of the proximal anal canal that has been termed splaying. Figure 28.9 shows a comparison of radiologic and ultrasound findings in a patient with rectal intussusception. Usually, a rectal intussusception is due to an enterocele of small bowel that progresses down the anal canal, but other abdominal contents such as the sigmoid colon (as in Fig. 28.9), omentum, and even the uterus can act in a similar way. Ultimately, rectal mucosa and muscularis may protrude from the anus, resulting in full-thickness rectal prolapse, but for obvious reasons such patients do not usually present to the gynecologist.
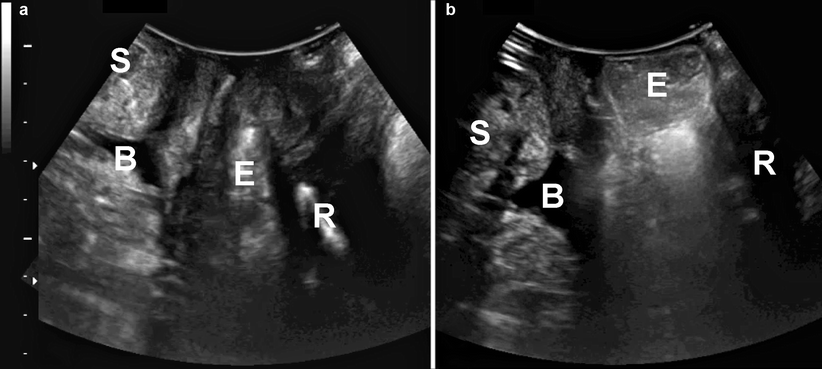
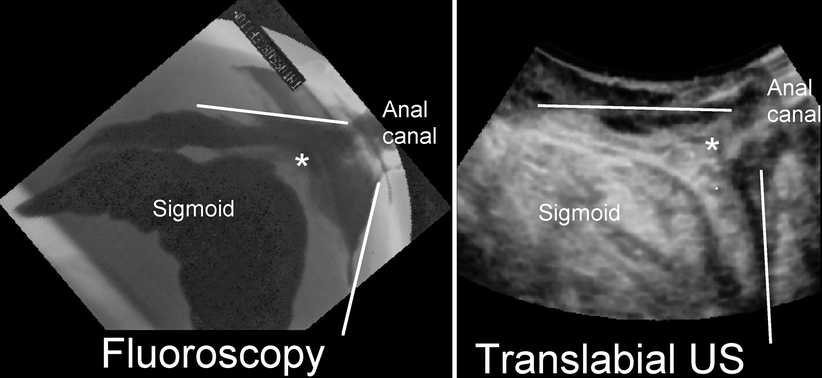

Fig. 28.8
Enterocele filled with small bowel, as seen in the midsagittal plane, at rest (a) and during Valsalva (b). S symphysis pubis, B bladder, R rectum, E enterocele

Fig. 28.9
A rectal intussusception (indicated by *) due to a sigmoid enterocele as seen on defecation proctogram (left) and translabial ultrasound (US; right). It is generally not possible to distinguish what part of the small or large bowel propels the intussuscipiens, although the coarse appearances of the intussuscipiens are suggestive of large rather than small bowel (Reprinted with permission from Perniola et al. [28])
When a rectocele or an enterocele is identified on translabial imaging, one may want to provide the patient with visual biofeedback. Demonstrating that straining during evacuation is obviously counterproductive (propelling feces not into the anal canal but into the vagina or propelling small bowel rather than feces into the anal canal) may help to modify behavior. Several recent studies have shown that ultrasound is tolerated much better than DP [28] and, of course, it is much cheaper. As a result, it is likely that ultrasound will replace DP as the initial investigation of women with defecation disorders. If there is a rectocele or a rectal intussusception/prolapse seen on ultrasound, this condition is likely to be found on x-ray imaging [28, 29]. In addition, anismus (an inability to relax the levator ani) may be present, which can be diagnosed qualitatively or by measuring the anorectal angle at rest and during straining, although often it is not clear whether observations on diagnostic imaging bear any relation to events during defecation or to its true incidence in such patients.