6 Radiesse® / Radiesse® with lidocaine
Summary and Key Features
• The ideal soft tissue filler for facial augmentation should be non-allergenic, and provide a natural look and feel
• The ideal soft tissue filler should require minimal downtime and exhibit few side effects
• Fillers can be permanent or temporary
• Calcium hydroxylapatite (CaHA) is well suited for facial augmentation and contouring
• CaHA has demonstrated a favorable safety profile
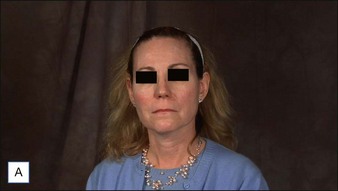
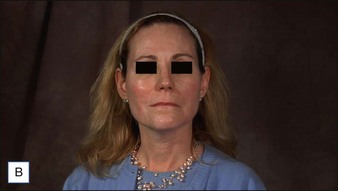
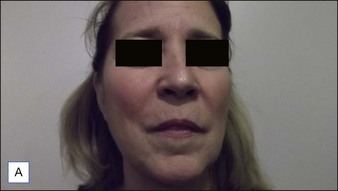
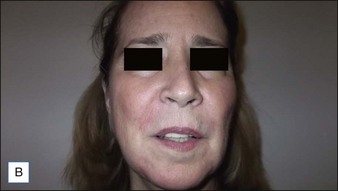
• CaHA longevity is approximately 15 months in active tissues of the face and may approach 24 months in more static locations
• CaHA is approved by the US Food and Drug Administration for correction of moderate to severe facial lines and folds and correction of soft tissue loss from HIV lipoatrophy
• Unlike collagen or hyaluronic acid filler materials, CaHA injection does not require overcorrection
• CaHA particles become fixed in position over time, do not migrate, and take on the natural characteristics of the soft tissue
• CaHA filler showed a high overall level of patient satisfaction, a remarkable safety profile, and clinical longevity