1. The potential risk of treatment with any form of radiation of a benign, nonlife-threatening disease must be recognized. Ionizing radiation therapy may be considered if other safer methods have not succeeded in alleviation of the condition and if the consequences of no further treatment are unacceptable
2. It must remain the prerogative of the physician to have available for use any form of therapy – radiation, drugs, or others – in which the benefits accruing to the patient from its use are considered to outweigh the risks inherent in its use
3. Infants/children should be treated with ionizing radiation only in exceptional cases
4. Direct irradiation of the skin areas overlying organs that are particularly prone to late effects, e.g., thyroid, eye, gonads, bone marrow, and breast, should be avoided
5. Medical practitioners using ionizing radiation should be adequately trained in both the practical and theoretical aspects of radiation therapy and protection
6. Meticulous radiation protection techniques should be used in all instances
7. The less penetrating X-ray qualities, e.g., Grenz rays, offer a wider margin of safety
8. Laboratory and epidemiologic studies should be initiated and/or continued to fill the gaps in our knowledge of the effects of ionizing radiation at the doses used in the past and currently
Even nowadays the use of RT for nonmalignant skin disorders can and should be based on the assumption that the FDA criteria have been fulfilled, other standard therapies for these conditions have been proven either ineffective or less effective than RT, special target cells and mechanisms are known, possible contraindications to the use of RT do not exist, the RT equipment and machines are well surveyed and calibrated regularly, and internationally accepted standards and appropriate RT guidelines and protection measures are applied on a routine base [9].
4.2 Use of Appropriate RT Techniques
The specific details of RT physics and technical aspects for the treatment of benign skin disorders are described in more details in another chapter of this book (Chap. 2, this book). In general for all the different skin disorders addressed in this chapter, specific anatomical considerations are most relevant for the targeting of the relevant cells and tissues, as the optimal choice of the appropriate RT technique will warrant achieving sufficient surface coverage and penetration depth. These anatomical aspects are summarized in Table 4.2.
Table 4.2
Anatomical extension and target depth for benign skin conditions normal skin (mm) nonmalignant cutaneous
Type of normal skin layer | Depth (mm) | Nonmalignant condition (examples) | Extension (mm) |
|---|---|---|---|
Epidermis | 0.02–0.25 | Dermatitis/eczema | 0.8–2.1 |
Epidermis | 0.04–0.5 | Psoriasis vulgaris | 0.7–3.2 |
Epidermis | 0.25–1.0 | Chronic lichen simplex | 1.1–4.4 |
Hair follicle | 0.5–3.5 | Folliculitis, acne | 3.0–5.0 |
Sweat glands | 1.0–3.0 | Benign skin tumors | 10.0 |
Deep fascia/structures | 2.0–10.0 | Hypertrophic scars or keloids, Dupuytren’s disease (hand palm), Ledderhose disease (foot sole) | 1.0–30.0 |
Figure 4.1 illustrates the typical composition of the skin and subcutaneous tissue and the different depths of target cells within the skin amenable to ionizing radiation.
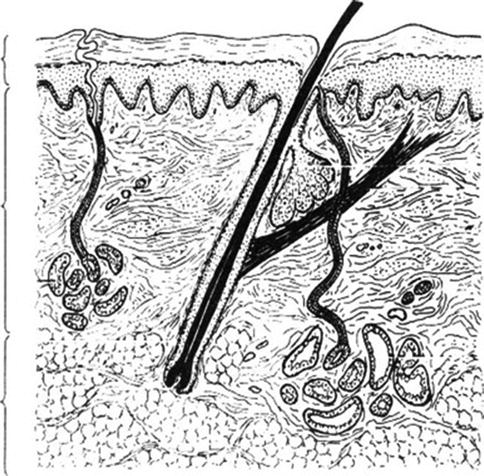

Fig. 4.1
Composition of skin and target cells for radiotherapy
4.2.1 RT-Dose Planning
Instead of using the former dosimetric concept of surface dose (SD) and half-value-layer (HVL), i.e., the depth at which the radiation dose reaches 50 % (D½), nowadays the total superficial spread and full depth of the treated lesion, i.e., the true dimensions of the target volume, is defined as the “target volume,” and then the dose is calculated to reach at least 90 % at the deepest portion of the lesion or of the defined target volume. However, this requires a “translation” of the former dose concepts into the newer ICRU 50/62 concepts using the reference point or isodose concept with specification of the minimum and maximum and the reference dose within the target volume. Thus, the extension of disease process determines the size of the target volume to be treated (planning target volume = PTV/clinical target volume = CTV) and the optimum type and energy of the radiation to be chosen (either being X-rays/linac electrons or brachytherapy). Depending on the dimensions of the target volume and the normal tissue at risk that have to be protected, different radiation energy techniques have to be applied [10].
4.2.2 Grenz Ray Treatment (GRT) (5–20 kV)
This very superficial application of ionizing radiation is the preferred RT technique for the treatment of the most superficial skin regions and benign skin conditions and considered as a first RT option in the most superficial cutaneous disorders like psoriasis or eczema. A specific chapter in this book has been dedicated to this technique (Chap. 5, this book).
4.2.3 Soft and Superficial X-Rays (SXR) (>20–100 kV)
The slightly deeper penetrating X-rays with energies between 20 and 100 kV are more suitable for disorders with skin involvement extending at least to the subcutaneous tissue layer, especially in the regions of the hand, palms, or foot soles. This X-ray quality is often applied for treatment of lymphocytoma cutis or recalcitrant keratotic eczematous disorders or other resistant or recurrent thicker dermatoses. GRT would not be sufficient in these clinical situations.
4.2.4 Deep X-Rays (DXR) (>100–300 kV)
The deep penetrating X-rays applied either by orthovoltage machines or by low-energy electrons (3–6 MeV) from modern linear accelerators are implemented for lesions which arise from the skin or subcutaneous tissue and are able to penetrate into the deeper tissue structures neighboring the skin layer or arise from the deeper tissue structures and extend into the skin surface layers. Most of these conditions address the hyperproliferative disorders like keloids or palmar and plantar fibromatosis or rarely the warts on extremities. SXR or even GRT would be insufficient RT techniques for these disorders.
4.2.5 Dose Limitation and Possible Cancer Induction
To avoid late effects of the skin and subcutaneous tissue including severe fibrosis or secondary tumor induction, it has been generally recommended to limit the total lifetime radiation dose for soft X-rays to 12 Gy and the total dose of Grenz rays to 50 Gy/per field and/or anatomic region. However, so far only very few case reports exist which have linked the application of dermatologic radiotherapy with the increased incidence of skin cancer, even if the radiation dose is given in the early childhood (examples: 11–13).
One of the best examined groups worldwide addressing the question of carcinogenesis after X-ray exposure derives from Scandinavia [11]: a large-scale study investigated the potential side-effects after exposure to GRT on 14,140 individuals who were treated with radiotherapy for eczema, psoriasis, and warts. After a minimum follow-up of 15 years, only in 58 patients (0.4 %) a malignant skin tumor was diagnosed, the earliest more than 5 years after GRT: 19 patients had developed malignant melanomas and 39 other malignant skin tumors. However, in the same period the expected number of incidental melanomas was 17.8 patients and that of other malignant skin tumors 26.9 patients. None of the patients with melanomas and only eight of the patients with other malignant skin tumors had received GRT at the site of the tumor, and six of these eight patients had also been exposed to other known carcinogens, like psoralen ultraviolet applications. Thus, the authors concluded that the available clinical data suggested that even doses up to 100 Gy per field and lifetime are not associated with any significant increase in side effects or tumor induction.
Similarly, it has been concluded, that in most cases of localized radiation exposure for therapeutic reasons, the cancer risks estimated by the effective-dose method may well overestimate the true risks by about one order of magnitude, yet in other cases even may underestimate it. The authors proposed a method using the organ-specific risk factors which is more suitable for the individual radiation treatment planning [12]. In addition Janssen et al. have proposed a method to calculate the risk of cancer induction in various realistic nonmalignant conditions including Dupuytren’s disease [13].
4.3 General Rules for Application of Radiotherapy
The use of RT for benign skin disorders is a safe form of therapy provided that appropriate safety guidelines are adhered to and that the prescribing physician has received adequate training and preserves clinic skills to recognize the various diseases and knows specific treatment options and undergoes continuous special training [14].
In addition to the general recommendations of the FDA (see Table 4.1), the following practice rules for the irradiation of benign skin diseases are emphasized [6, 15, 16]. Specific guidelines have also been published by the German Cooperative Group for Radiotherapy of Benign Disorders (GCG-BD) (Table 4.3).
Table 4.3
General practice rules for the application of radiotherapy
1. Diagnosis of the disorder must be clearly established (if possible by biopsy) |
2. There should be an established mechanism of action or defined target cells on which ionizing radiation interacts to expect an improved outcome |
3. Radiation treatment should be started at the appropriate clinical situation of the specific disease, especially after failing previous treatments (e.g., after surgery for keloids or topical medical treatment for eczema) |
4. Patients have to provide a clear record about possible previous RT treatments and received RT doses per body site should be precisely known if appropriate |
5. Certain defined RT dose limits are not to be exceeded per RT field and lifetime |
6. Use of additional topical treatments prior to and parallel to RT can induce irritating effects and/or reduce the intended X-ray effect |
7. Known radiosensitive organs (e.g., eyes, thyroids, breasts, gonads) should be carefully protected by means of absorbing materials (e.g., lead foils) or adequate RT techniques (such as beam direction etc.) |
8. Use of RT in children and young adults for benign skin disorders is very rare |
As the overall implementation of RT for nonmalignant skin disorders has decreased in the past decades, the order of its use has also changed. In earlier surveys, some disorders like acne, warts, and hidradenitis were much more often irradiated than eczematous conditions. In contrast, the emphasis in recent years has been on the use of superficial X-rays for difficult to treat clinical situations and for chronic eczemas [17, 18] and the use of RT for hyperproliferative disorders such as keloids [19] and palmar or plantar fibromatosis [20].
The following clinical sections discuss the various specific benign skin conditions in which radiation therapy may still be helpful, starting with those dermatoses in which Grenz ray treatment is likely to suffice and ending with the hyperproliferative disorders which need RT techniques with deeper penetration, such as DRT and low-energy electrons.
4.4 Radiotherapy for Eczema and Eczematous Dermatitis
4.4.1 General Aspects
Eczematous dermatitis is a common condition that may negatively interfere with professional and leisure activities, the social function, recreation, and sleep activities of afflicted persons. Its long-term persistence and especially the accompanying pruritus may be very stressful and frustrating. The attempts to relieve the itch by scratching simply worsen the rash, thereby creating a vicious circle and secondary problems. Clinically various types of eczemas have to be differentiated. The most common and best characterized type of eczema, the atopic dermatitis, appears to be increasing in incidence over the past decades. Other more common eczematous dermatoses must be accurately diagnosed, particularly the allergic dermatitis and irritant contact dermatitis, as possible improvement and even complete resolution strictly rely on the appropriate diagnosis and avoidance of pertinent triggering factors.
The therapy should be directed at limiting the itching and allowing the long-term repair of the damaged skin and decreasing the surrounding inflammation when necessary. Thus, the principles of treatment include careful general skin care (including appropriate skin cleaning and moisturizing), patient’s education about prophylaxis and strict avoidance of specific irritants, use of different methods of skin hydration and lubrication, and when necessary the temporary and adequate use of topical corticosteroids. The recommended systemic medication includes antihistamines and corticosteroids for certain clinical situations, but is not generally advocated for therapy of chronic eczematous dermatitis. If the pruritus does not respond to local or systemic measures as described above, other diagnoses, such as bacterial overgrowth or viral infections, should be excluded.
Radiotherapy as a treatment option is available for the refractory recurrent chronic eczemas, but should be reserved for more unique situations and always requires an interdisciplinary consultation between medical specialists including allergist, dermatologist, and radiation therapist/oncologist. Radiation treatment should be performed by the radiation therapist and evaluation again by the interdisciplinary team.
4.4.2 Rationale and Technique of Radiotherapy
In general, very low-energy X-rays are sufficient to reach the sensitive cells which create the eczematous lesions. Thus, in most cases Grenz rays with 10–20 kV are a suitable technique. However, in some chronic, long-standing eczematous conditions, especially palms and soles, more penetrating radiation qualities of 20–50 or even 100 kV, are much more effective because of better penetration depth [16, 17]. If Grenz rays are not available, linear accelerator-based therapy with electrons of the lowest available energy (e.g., 3–5 MeV) plus 5 mm bolus would be the preferred technique. The main rationale of using radiotherapy as treatment is the known positive effects of low radiation doses on the inflammatory process and the affecting enzymes, cytokines, and peripheral mononuclear blood (PMNB) cells, especially the lymphocytes which are the cellular component of the inflammatory process [21–25].
The effects of X-rays on the eczematous lesions are probably mediated by a decrease of epidermal Langerhans’ cells after irradiation [26]. Different RT concepts for soft X-rays in eczemas have been studied by Goldschmidt [27]; they could demonstrate that single doses of 0.4 Gy and lower are less effective and repeated fractions are required to compensate for the reduced effect of lower doses; however, no further improvement was achieved with higher single doses of 0.6–1 Gy. The typical RT dose recommendations are summarized in Table 4.4. In general, a typical radiation course consists of 6–12 fractions of 2 Gy Grenz rays (with 20 kV energy) or 1 Gy soft X-ray (up to 100 kV) two or three times per week.
Table 4.4
Dose recommendations for chronic eczemas
D½ | 1–3.0 mm |
Energy (kV) | 10 or more |
Filter | None or 0.4 mm Al |
HVL | 0.1 or 0.2 mm Al |
Single dose | 0.5–2 Gy |
Total dose | 3–12 Gy |
Interfraction interval | 3–6 days |
Fractionation | 1–3×/week for 2–4 weeks |
4.4.3 Clinical Experience and Results
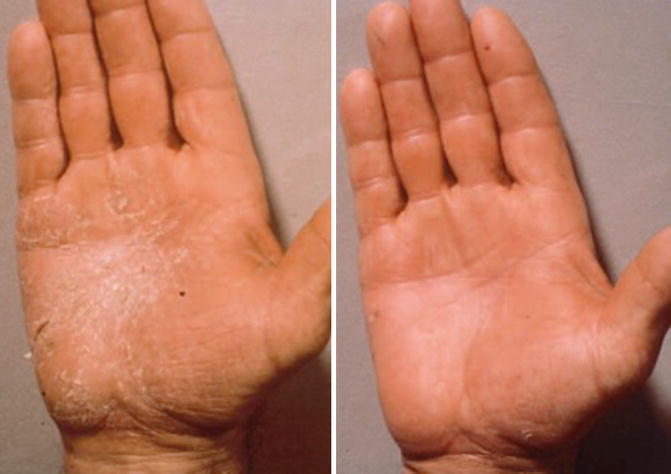
The early clinical studies reported favorable response rates for hyperkeratotic eczemas and chronic lichenified eczemas. In these studies usually single radiation doses of 0.75–1.0 Gy were administrated at weekly intervals over a period of 3–4 weeks up to a total radiation dose of 4–6 Gy [3]. Few other clinical studies suggested the application of lower single doses in the range of 0.5–0.75 Gy once per week or more protracted in intervals of every 2 weeks for a limited number of two to three exposures and a total radiation dose of 1–3 Gy [28]. With regard to randomized studies and the comparison with a placebo control, the following clinical investigations are important: in one investigation, 24 patients with chronic symmetrical constitutional eczema of the hands were treated with superficial X-ray therapy applying three fractions of 1 Gy in intervals of 21 days (total dose 3 Gy) to one hand, while the other hand received a placebo treatment. A significantly better therapeutic result was recorded on the hand that received X-ray treatment [16]. The same group explored in a double-blind clinical study the difference between the use of superficial X-ray therapy (>20–100 kV) and Grenz ray therapy (10–20 Gy). As to be expected, they found that using conventional superficial X-rays (up to 100 kV) and total dose of 3 Gy applied in three fractions of 1 Gy was superior to Grenz rays (20 kV) and a total dose of 9 Gy applied in three fractions of 3 Gy with fractions spaced 21 days apart [17].
Recently a few case studies and one clinical series have been published which support the successful concept of superficial low-dose radiotherapy when using the megavoltage equipment: Stambaugh and coworkers [29] reported a patient who was refractory to multiple forms of topical and systemic agents, but achieved complete resolution of severe dyshidrosis within 1 month following low-dose radiation therapy; the durable response even allowed withdrawal of oral steroids after 6 weeks without flare of disease and with the patient remaining free of medication at the 6-month interval. Walling and coworkers [30] published a case study of a 48-year-old dermatologic surgeon with frictional hyperkeratotic hand dermatitis (FHHD) – an unusual form of chronic eczema related to repeated frictional trauma. The sudden eruption of these lesions was completely resistant to all topical and protective treatment modalities, but finally the lesion responded to a total of six sessions of 1 Gy Grenz ray treatment (total dose 6 Gy). Despite continuous work, no relapse occurred for over 4 years since completion of therapy.
The latest published clinical series from a Swiss group [31] reported long-term outcome of 22 patients suffering from therapy-refractory eczema and six patients with psoriatic lesions of the palms and/or soles. Lesions received twice weekly either a single dose of 1 Gy up to a median total dose of 12 Gy or 0.5 Gy up to a median total dose of 5 Gy. A total of 88 regions were treated, 49 with 1 Gy and 39 with 0.5 Gy single dose. Eight symptoms were scored from zero (absent) to three (severe), resulting in a possible sum score of 24 points. Patients’ rating was also recorded (worse/stable/better/complete remission). After a median follow-up of 20 months, the sum score had decreased from 15 [6–20, 32–34] before RT to 2 (0–16) at the end of RT and to 1 (0–21) at last follow-up, respectively. The improvement was highly significant in both treatment regimens. Good remission was also stated by the patients in 83 of 88 localizations. From these data the group recommended a single dose of 0.5 Gy twice weekly up to a total dose of 4–5 Gy.
In summary, the major limitation of RT for eczematous lesions is that it may be repeated only for a few times until reaching the still very cautiously defined lifetime dose limit. Thus, it is important to note that ionizing radiation is rarely advisable for the chronic atopic (constitutional) eczema because of the high tendency to local recurrence. The lichen simplex chronicus often responds quickly to radiation exposure; the antipruritic effect of ionizing radiation in this dermatosis and in other skin diseases is quite striking.
4.5 Radiotherapy for Psoriasis and Psoriatic Conditions
4.5.1 General Aspects
Psoriasis is a noncontagious chronic inflammatory disease of the skin caused by an accelerated growth cycle of skin cells and is mediated by the immune system. The severity varies from small localized patches to the complete body coverage. Clinically two major groups, the nonpustular type and the pustular type psoriasis, can be distinguished.
The nonpustular psoriasis is differentiated into the plaque–like psoriasis (chronic stationary form) which is the most common form and affects 80–90 % of all patients; in the plaque-type psoriasis, the skin rapidly accumulates and thickens at distinct locations providing a silvery-white appearance. The plaques commonly occur on the elbows and knees but may also affect any other area of the body. Another nonpustular type is the erythrodermic psoriasis characterized by a widespread inflammatory and exfoliative skin pattern which can be accompanied by severe symptoms like swelling, itching, and pain and severe complications due to the loss of the skin barrier function and thermoregulation.
The pustular psoriasis is characterized by raised skin bumps filled with noninfectious pus; the surrounding skin is red and tender. This type of psoriasis commonly is localized and affects the hand and feet (i.e., palmoplantar psoriatic pustulosis), but can be also more generalized (i.e., palmoplantar pustular psoriasis) and may develop different other types like annular pustular psoriasis, acrodermatitis continua, and impetigo herpetiformis (see Fig. 4.4).
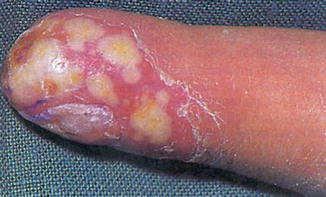

Fig. 4.4
Pustulous finger psoriasis
One type of psoriasis is the inverse psoriasis which typically affects the skin folds particularly between the thigh and the groin, the armpits, and underneath the breasts, the panniculus, or other skin folds; it is aggravated by skin friction, sweat, or liquids and may be prone to fungal infections. Another type is the guttate psoriasis which shows numerous small scaly, red to pink drop-like lesions; these psoriatic spots may cover large parts of the body, primarily the trunk and less often the extremities and the scalp; it may be preceded by a streptococcal infection mostly originating from the pharynx.
A special type of psoriasis which is relevant for radiotherapy indications affects the nails of fingers and toes (nail psoriasis): it produces a variety of characteristic changes including the discoloring underneath the nail plate, the pitting of the nails, the appearance of lines passing across the nails, the loosening and crumbling of the nail (onycholysis), and the thickening of the skin underneath the nail (see Figs. 4.5 and 4.6).
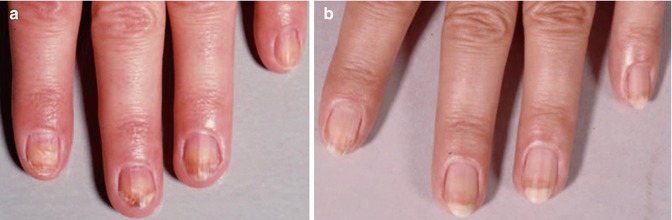
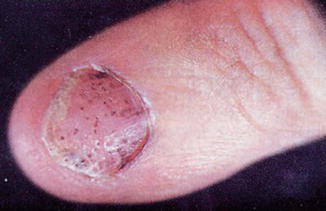

Fig. 4.5
Psoriasis of fingernails in a 46-year-old women (a) before and (b) 8 months after treatment, 12 Gy total dose with 12 kV

Fig. 4.6
Subungual finger psoriasis
In summary, there are five different patterns of psoriasis appearance: the most common is the plaque type; the other types are the guttate, inverse, pustular, and erythrodermic type.
In 10–40 % of all affected patients, an inflammation of various joints can occur which is known as psoriatic arthritis. The specific cause of psoriasis is not well understood, but a genetic component, a local injury, and environmental factors have been suggested which can aggravate psoriatic affections including stress factors or withdrawal of steroids. Despite the various treatment options available, none is really suitable to provide lifelong relief. Treatment of psoriasis remains a general and individual challenge. In the acute situation, most often topical ointments are applied containing corticosteroids. Radiotherapy is limited to refractory cases in specific anatomical locations.
4.5.2 Rationale and Technique of Radiotherapy
Several decades ago Grenz ray treatment (GRT) was widely used to treat psoriatic lesions, but nowadays – with many options of local and systemic medications available – X-ray therapy currently is used only as a last resort in difficult-to-treat-situations like recalcitrant localized lesions in psoriasis of the scalp or psoriasis of the nails. Moreover, in view of the tendency of psoriasis to recur, the current opinion is to limit X-ray therapy only to very severe and refractory cases. In contrast to other psoriatic lesions, the palmoplantar pustular psoriasis is not considered a valid indication for the use of radiotherapy [35].
In former clinical studies it has been shown that RT technique is of some importance. While one group found no difference between conventional superficial X-ray therapy and Grenz rays in over 70 % of the patients [36], other groups have observed a superior and longer-lasting effect of deeper penetrating superficial X-rays as compared to Grenz rays; however, the beneficial effect is mostly dependent on the complete physical coverage of the treated lesion. If properly applied radiation therapy is not associated with any major side effects and it remains still to be seen whether the local application of antimetabolites or long-range psoralen-ultraviolet A (PUVA) treatments will develop fewer sequelae than judiciously used X-ray therapy. Today previously accepted X-ray treatments are nowadays considered a contraindication to PUVA therapy in view of the potential inherent increase in the frequency of treatment-related skin cancers by PUVA itself.
Regarding the use of an appropriate RT technique, it is important to estimate the nail thickness and to know the specific transmission of the chosen X-rays through normal and diseased nails before irradiating any psoriatic nails. Although low-energy Grenz rays (with up to 20 kV energy) may be tried in psoriatic nails of normal thickness [37], it has been shown that they are much less effective in the thickened diseased finger or toe nails [38]. Since most psoriatic nails are relatively thick, higher energy X-rays (up to 100 kV) should be preferred. If only electrons from linear accelerators are available, the lowest possible energy (3–5 MeV) and adaptive bolus material with 5–10 mm thickness should be chosen to limit the penetration depth to 5–10 mm. Dose recommendations for psoriasis of the nails are summarized in Table 4.5.
Table 4.5
Dose recommendations for psoriasis of the nails or scalp
D½ | 1–5 mm |
kV | 10 or more |
Filter | none or 0.4 mm Al |
HVL | 0.1 or 0.2 mm Al |
Single dose | 0.5–2 Gy |
Total dose | 3–12 Gy |
Fractionation | 1–3×/week for 2–4 weeks |
4.5.3 Clinical Experience and Results
Good clinical responses were reported in over 50 % of irradiated patients, when treated with three fractions of 1–1.5 Gy at weekly intervals; the fingernails, nail matrix, and the periungual areas should be always included in the irradiated fields [39]. Finnerty described three patients treated with six to eight doses of 0.5–0.75 Gy with a total dose of approximately 4–6 Gy; all treated nails were cleared after several months [40]. Kouskoukis and colleagues suggested a single dose 1 Gy at weekly intervals up to a total dose of 4–5 Gy with which they achieved clinical remissions lasting for several months up to many years [41]. In accordance with other reports in the literature, the recommended dose schedule is summarized in Table 4.5, i.e., 6–12 fractions of 2 Gy soft X-ray two times a week or 1 Gy three times a week. Dose recommendations for psoriasis of the scalp are the same as for the nails.
4.6 Lymphocytoma Cutis (Pseudolymphoma)
4.6.1 General Aspects
Lymphocytoma cutis is a rare type of pseudolymphoma that is also defined as “cutaneous lymphoid hyperplasia” or “lymphadenosis benigna cutis.” Early lesions mostly start in the third to fourth decade, with a male to female ratio of 3 to 1 and a white to black race ratio of 9 to 1.
Clinically the disorder can occur in a more circumscribed or localized or a widespread or disseminated form. It appears to be a reactive process of “blood cell cancer” developing in the skin; however, it behaves more as a benign tumor in a rather harmless manner [42, 43].
Commonly no cause can be attributed to the disease itself, but some may act as trigger process which include contact with foreign agents (proteins) like reaction to tattoo dyes, reactions after exposure to insect bites, stings of mosquitoes and spiders, vaccination agents, desensitization injections, or acupuncture procedures; some other skin trauma like piercing or infection with Borrelia burgdorferi (Lyme disease), varicella zoster (chickenpox), and human immunodeficiency virus (AIDS virus) are also considered as trigger factors.
Clinically the lesions present either as non-itching soft and doughy or more firm nodules of red to brown or red to purple color and occasionally with a scaly or crusty surface. About two thirds of the lesions develop on the face and another third on the chest and upper extremities. Over 70 % appear as single nodules or local group of small lesions, while numerous or disseminated lesions are rather uncommon.
Skin biopsy reveals a local inflammatory process with mixed B and T lymphocytes and benign immunohistochemistry. Typical features of malignant lymphomas are absent, e.g., tingible bodies, higher proliferation rate, positive gene rearrangement, and cells that stain with CD10+ and Bcl6+ outside follicles and Bcl2+ within follicles; lesions can mimic B-cell lymphomas such as follicle center lymphoma, marginal zone B-cell lymphoma, or large B-cell lymphoma. Classification into B-cell or T-cell and by its cause, if any is identified, has been proposed. Often times the more general term of “pseudolymphoma” is used. Table 4.6 summarizes the two classes and subtypes.
Table 4.6
Classification and subtypes of lymphocytoma cutis/pseudolymphomas
B-cell lymphocytoma cutis |
Primary or Idiopathic cutaneous B-cell pseudolymphoma |
Borrelial lymphocytoma cutis |
Tattoo-induced lymphocytoma cutis |
Post-zoster scar lymphocytoma cutis |
Persistent nodular arthropod-bite reactions |
Lymphocytoma cutis caused by antigen injections/acupuncture |
Lymphomatoid drug eruptions |
Acral pseudolymphomatous angiokeratoma |
T-cell lymphocytoma cutis |
Primary or idiopathic cutaneous T-cell pseudolymphoma |
Lymphomatoid drug reactions |
Lymphomatoid contact dermatitis |
Actinic reticuloid (chronic actinic dermatosis) |
Anticonvulsant-induced pseudolymphoma |
Persistent nodular arthropod-bite and scabies reaction |
Acral pseudolymphomatous angiokeratoma |
Other than distinguishing this condition from cutaneous lymphoma, it is also important to consider other alternative diagnoses like Jessner lymphocytic infiltrate, cutaneous lupus erythematosus, and plaque type of polymorphous light eruption. Specific features seen in the biopsy specimen may be the only way to determine the correct diagnosis in some cases.
4.6.2 Rationale and Technique of Radiotherapy
Radiotherapy is only one of many options to treat lymphocytoma cutis. The first approach is the detection and removal of any potential cause or offending agent. Watchful waiting can be accepted to await spontaneous regression unless the patient develops symptoms or is disturbed by the esthetic appearance. Small series have been successful with the following agents: topical steroids or intralesional steroid injections, topical tacrolimus or hydroxychloroquine, or surgical methods such as local excision or cryotherapy. The use of RT with small doses of superficial X-rays has been shown to be effective especially for localized and circumscribed lesions which do not respond to primary treatments, however, with early lesions being far more radiosensitive and responsive to RT than older lesions. The recommended RT schedule is presented in Table 4.7.
Table 4.7
Dose recommendations for lymphocytoma cutis/pseudolymphoma
D½ | 1–15 mm |
kV | 50–100 or more |
Filter | 0.4–2.0 mm Al |
HVL | 0.2–1.6 mm Al |
MeV | 3–5 |
Bolus | 5–8 mm |
Single dose | 1–2 Gy |
Total dose | 2–12 Gy |
Fractionation | 2–4×/week |
4.6.3 Clinical Experience and Results
Benign lymphocytomas respond to very small RT doses of 0.75–1 Gy in two fractions within 1 week up to a total dose of 1.5–2 Gy or a single treatment with 1.5–2 Gy or single doses of 3 Gy at 3–4-week intervals up to 12 Gy total dose [28, 44]. Goldschmidt and Sherwin reported successful treatments with single doses of 1.5–2.5 Gy at 1–3-week intervals in one to three sessions [3]. Some other groups have suggested higher single doses of 5 Gy up to a total dose of 25 Gy. In one other series, multiple lesions on the face were treated with 15 Gy in five fractions of 3 Gy over a period of 5–7 days with excellent cosmetic results [45].
4.7 Radiotherapy for Keloids and Hypertrophic Scars
4.7.1 General Aspects
Keloids or “keloid scars” represent overgrowth of granulation tissue at the site of a healed skin injury where collagen type 3 is replaced by collagen type 1; they form as a result of an aberrative physiologic wound healing process after insult to the deep dermis. Depending on its maturity keloids are composed of either type III (“early type”) or type I (“late type”) collagen; they should not be confused with hypertrophic scars, which also raise above skin level but do not show lateral growth beyond the boundaries of the original wound.
Keloids do affect both sexes equally, although the incidence in younger females has been reported to be higher than in the younger male, which is probably reflecting the higher frequency of earlobe piercing and other piercings among women. Interestingly, there is a significantly higher frequency of occurrence in highly pigmented populations; especially Africans and Afro-Americans are at increased risk of keloid formation.
Keloids present as “benign tumor-like lesions” which are firm, rubbery lesions or fibrous nodes that vary in color from pink to flesh-like or red to dark brown. Histologically they are characterized by atypical fibroblasts with excessive deposition of extracellular matrix components, especially collagen, fibronectin, elastin, and proteoglycans; they usually contain relatively acellular centers and thick, abundant collagen bundles that form nodules in the deep dermal portion of the lesion.
By causing pain, local inflammation, pruritus, and contractures, the excessive scarring can significantly affect the patient’s quality of life, both physically and psychologically. Severe cases may interfere with the movement of the skin and even joints can be affected. Local infection(s) and/or bleeding are complications which require additional surgery. In visible areas (e.g., face, ear, breast etc.) the personal esthetic is heavily disturbed and may lead to severe personal stigmatization, isolation, discrimination, and restricted lifestyle of the patient which can severely affect the overall quality of life.
So far no universally accepted treatment protocol has been established. Some modalities have been introduced several decades ago; others have been introduced more recently. Surgery is the treatment of choice, but also compromised by high relapse rate of 50–80 % if used as single modality. Additional therapies were introduced as preventive strategies after local surgery including radiation therapy, pressure therapy, cryotherapy, intralesional injections of corticosteroids, interferon, fluorouracil, topical silicone, and pulsed-dye laser treatment. Thereby the recurrence rate of 50–80 % after surgery was reduced to 50 % with intralesional steroid therapy, while external RT reduced the recurrence rate to 12–28 %.
4.7.2 Rationale and Technique of Radiotherapy
The use of RT belongs to the long-term established treatments despite lack of randomized studies but owing to large patient populations treated and a convincing radiobiological rationale as several radiosensitive target cells and biological mechanisms available which support the therapeutic rationale and are summarized in Table 4.8
Table 4.8
Radiosensitive targets and mechanisms for hyperproliferative disorders
2. Induced free radicals impair proliferative activity of fibroblasts [48] |
3. Interference with growth factors, especially PDGF and TGF-beta [49] |
4. Reduction of activated monocytes and macrophages interacting with the inflammatory process and myofibroblast proliferation [50] |
5. Similar radiosensitive target cells/mechanisms found for prophylactic RT: |
In keloid relapses after surgical excision [53] |
In relapses of recurrent pterygium [54] |
Different RT techniques have been reported and applied in the past: low-energy orthovolt radiotherapy, high-energy external beam electrons from linear accelerators, and remote-control afterloading brachytherapy through implanted catheters. There has been a long debate about the optimal timing and RT dose concept.
RT is rarely considered as primary treatment for keloids, but very effective when combined with surgery [28]. Clinical response improves if keloids are treated at the earliest time, generally considered to be within 72 h of excision. A study showed that RT is less effective in keloids older than 6 months. In contrast, Enhamre and Hammar et al. found no correlation between therapeutic results and time interval between excision and irradiation, reporting 88 and 99 % good results, respectively [55].
Different fractionations, single and total doses, have been applied ranging from 2 to 20 Gy, administered over a period of 1 day to 2 weeks [56–61]. A minimum isoeffect time-dose line for postoperative keloid control was seen with total doses of 9–10 Gy delivered over 1 week or 15 Gy delivered over 2 weeks. Higher total doses or high single doses of 10 Gy are prone to late effects like teleangiectasias [62].
Two meta-analyses have examined RT dose requirements. Kal and Veen [63] found a dose relationship with decreasing relapse rate as a function of the bioequivalent dose (BED): BED dose above 30 Gy resulted in a relapse rate below 10 % and no differences within high stretch tension sites. Recommended RT doses fulfilling this concept were 1 × 13 Gy and 2 × 8 Gy external beam RT or 27 Gy LDR brachytherapy [63, 64] undertook a radiobiological analysis of postoperative keloid RT. A 95 % long-term local control was achieved with three fractions of electron beam RT up to 18.3–19.2 Gy (for the earlobe) or 23.4–24.8 Gy (for other sites) total RT dose. Single fraction equivalent doses were 11.4 Gy (earlobe) and 14.5 Gy (other sites) [64]. The actual dose recommendations for keloids are listed in Table 4.9




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree