Procedural Skills
The following discussion presents some of the common dermatologic procedures performed in the office setting by advanced practice clinicians. Content is provided to serve as a guideline that is incorporated into the clinical judgment. Performing these, or any, procedural skills requires the development of competency to optimize patient safety and outcomes. Therefore, it is recommended that clinicians should:
Acquire essential knowledge of the procedure, indications, and complications. However, knowledge alone does not confer competency.
Obtain basic instruction for the skill, including observation.
Demonstrate the skill under the supervision of a trainer or experienced clinician until it can be performed in its entirety without any mistakes or concerns. This should be done in a variety of settings that simulate real patient care.
Perform continuous self-assessment, with patient and peer feedback and educational updates.
Document competency of skills, which is both valuable and required in some health care settings or by regulatory boards.
PUNCH BIOPSY
Author:
Theodore D. Scott, RN, MSN, FNP-C, DCNP
Description
Punch biopsy is a nonsterile procedure by which sampling of an endophytic skin lesion or full thickness of skin is performed for the purpose of histopathologic examination.
Indications
Small pigmented lesions of the skin (nevi or small melanomas)
Benign skin tumors (i.e., dermatofibroma, neurofibroma)
Vascular disease of the skin or subcutaneous fat
Superficial inflammatory or granulomatous diseases
Papulosquamous disease (i.e., psoriasis)
Connective tissue disorders (i.e., systemic lupus erythematosus, discoid lupus erythematosus)
Contraindications
Infection at the biopsy site
Superficial artery or nerve (i.e., Erb’s point) at biopsy site
Pigmented lesion larger than available punches
Equipment
Alcohol or chlorhexidine prep pads
Examination gloves and sterile gloves
Syringe, usually 3 mL or 5 mL
25G to 31G needle depending on site; 31G insulin syringes are helpful for noses and ears.
Lidocaine 1% or 2%, with or without epinephrine, depending on site
Sterile 4 × 4 or 2 × 2 gauge sponges
Baker-type biopsy punch or equivalent, commercially available in sizes 2 to 12 mm
Pickup forceps
Iris scissors or scalpel
Needle drivers
Monofilament nonabsorbable suture appropriate for the thickness of skin; 4-0 black nylon on a P-12 needle is useful for most punches.
Absorbable suture for larger punches (8 to 12 mm)
Formalin in normal saline specimen containers of appropriate size for the specimen prelabeled with the patient’s name and medical record number—never label after the fact. A biopsy for direct immunofluorescence should be placed in a container with Michel’s transport medium.
Hyfrecator for control of bleeding
Petrolatum-based ointment (like Vaseline or Aquaphor)
Adhesive dressing of appropriate size (check for allergy)
Preparation
Procedure details, risks, and alternatives are discussed with the patient. All questions are answered and informed consent is given by the patient.
All specimen containers to be used and histology requisitions are labeled and information is verified by patient.
After gloving, the area to be sampled is cleaned with alcohol or chlorhexidine.
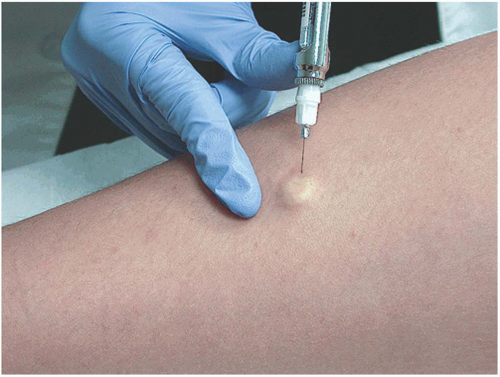
Lidocaine is injected intradermally below the lesion to be sampled; if done correctly, the lesion will be raised on a wheal (Figure 24-1).
Procedure
Sterile gloves are preferred for closure with sutures.
With the nondominant hand, stretch the skin perpendicular to the relaxed skin lines. This will result in an elliptical defect when the tension is released making for a much more cosmetically acceptable closure.
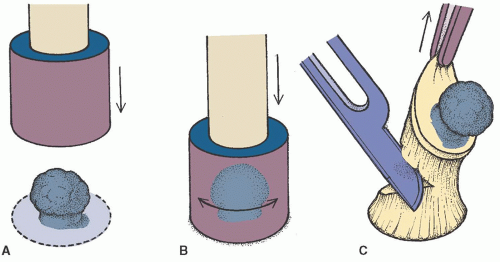
With the dominant hand, place the biopsy punch over the lesion and gently apply downward pressure while twisting the punch until you feel a slight pop and the punch goes through the dermis (Figure 24-2A and B).
Retract the punch from the defect. Gently lift the freed skin specimen with the pickups and cut or snip the bottom attachment with the subcutaneous fat (Figure 24-2C).
Place the specimen into a container and seal.
Blot biopsy site with gauze square and apply direct pressure to the site. Electrocautery with a hyfrecator may be used with caution. Proceed when hemostasis is complete.
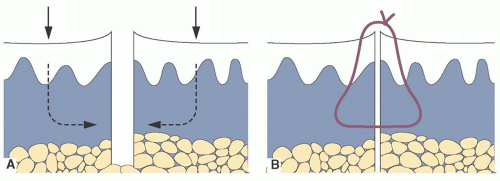
Suture the defect closed with interrupted sutures; larger defects may also require an absorbable deep cuticular suture to prevent a dead space at the wound base (Figure 24-3).
Apply petrolatum ointment to wound after hemostasis is achieved.
Apply adhesive dressing.
Give patient both verbal and printed aftercare instructions in their preferred language.
Anticipated Outcomes
Mild pain at biopsy site
Possible infection
Possible bleeding
Possible separation of the wound edges of punch biopsy
Scar at the biopsy site. Be sure to emphasize this point when you obtain informed consent.
Aftercare
Initial bandage may be left in place for 24 hours unless saturated with blood.
After 24 hours, bathe as normal and wash the biopsy site with gentle soap and water only.
After drying, apply petrolatum ointment to the suture line and bandage.
Stay out of oceans, lakes, and swimming pools until after the sutures are removed.
Return for suture removal as indicated
Face and neck in 3 to 7 days
Arms in 7 to 10 days
Trunk and legs in 10 to 14 days
Keep wound moist with petrolatum and covered for 1 to 2 weeks for optimal healing and best cosmetic results.
The patient should be educated about the signs and symptoms of infection, including redness, warmth, tenderness, and discharge. Contact information should be given in the event that this should occur.
Inform patient when and how they will receive the results of their biopsy.
Arrange for suture removal.
SHAVE BIOPSY
Author:
Theodore D. Scott, RN, MSN, FNP-C, DCNP
Description
Shave biopsy is a nonsterile procedure by which sampling of an exophytic or shallow endophytic skin lesion is performed for the purpose of histopathologic examination.
Indications
Raised lesions
Dome-shaped nevi and benign tumors
Nonmelanoma skin cancers
Contraindications
Infection at the biopsy site
Vascular lesion of unknown extent for depth (cavernous hemangioma)
Deep melanocytic lesions suspected for melanoma (only punch, excisional, or deep saucerization are indicated for these deep lesions).
Equipment
Alcohol or chlorhexidine prep pads
Examination gloves
Syringe, size as required (usually 3 cc or 5 cc)
25G to 31G needle depending on site. 31G insulin syringes are helpful for noses and ears.
Lidocaine 1% or 2%, with or without epinephrine, depending on site
Sterile 4 × 4 or 2 × 2 gauge sponges
DermaBlade or scalpel blade (no. 15)
Formalin in normal saline specimen containers of appropriate size for the specimen prelabeled with the patient’s name and medical record number—never label after the fact.
Aluminum chloride 20% solution (Drysol), Monsel’s solution, or hyfrecator
Cotton-tipped applicators
Petrolatum-based ointment (Vaseline or Aquaphor)
Adhesive dressing of appropriate size
Preparation
Same as for Punch Biopsy Procedure
Using the DermaBlade or scalpel blade, tangentially shave the lesion off the skin with a gentle side-to-side movement (Figure 24-4).
Place the specimen into a container and seal.
Blot biopsy site with gauze square and apply cotton-tip applicator saturated (not dripping) with the aluminum chloride or Monsel’s solution. Light electrocautery with a hyfrecator may be needed.
Apply petrolatum ointment to wound after hemostasis is achieved.
Apply adhesive dressing.
Give patients both verbal and printed aftercare instructions in their preferred language.
Anticipated Outcomes
Same as with punch biopsy
Aftercare
Same as for punch biopsy, except for suture removal
CLINICAL PEARLS
Avoid epinephrine on fingertips and penile tip.
Punch biopsies ≤4 mm can be left open to heal by secondary intention.
Alcohol and hydrogen peroxide are no longer used for routine wound care as they are toxic to the keratinocytes.
Neomycin, polymyxin, and bacitracin ointments (Neosporin) are not recommended as they are potential sensitizers and may provide little protection.
For all skin diseases other than pigmented lesions, a 4-mm punch will give the pathologist adequate material to make a diagnosis.
For pigmented lesions, select a punch that will completely sample the lesion and a slim margin of clear skin.
Never perform a shallow shave biopsy of a pigmented skin lesion to avoid the risk of transecting the pigmented lesion. The most predictive factor in the clinical course of melanoma treatment is the depth of invasion on initial biopsy. This information would be lost.
Never take a small punch or several small punches of a large pigmented lesion. A small punch of a large lesion will not provide the most accurate measurement of the depth.
If a pigmented lesion is too large to sample comfortably in your clinic, arrange expedient referral to a provider skilled in dermatology or surgery.
Avoid Monsel’s solution and silver nitrate in cosmetically sensitive areas as they can leave a permanent pigmentation.
SKIN TAG REMOVAL
Author:
Kathleen E. Dunbar Haycraft, DNP, FNP/PNP-BC, DCNP, FAANP
Description
Procedure to remove benign skin tags by various methods
Indications
Symptomatic (tender, bleeding, or itching)
Cosmetic concerns
Equipment
Depending on the method used:
Alcohol swabs
Gauze 4 × 4
Lidocaine, or topical anesthetic
Small sharp scissors (gradle)
Forceps (optional)
Liquid nitrogen
Hyfrecator
Preparation
Procedure details, risks, alternatives, and recurrence are discussed with the patient. All questions are answered and informed consent is given by the patient.
Advise patients that this may be considered a cosmetic procedure and not covered by insurance.
If you have any doubt as to the benign nature of the lesion, send it for biopsy.
Cleanse the area with antiseptic preparation and allow it to dry.
Consider anesthesia options: ice for 1 minute prior to removal; a brief spray of liquid nitrogen (LN2); topical anesthetic; lidocaine injection (more painful than the actual removal); and no anesthesia, which is very common for clipping, hyfrecation, and cryotherapy.
Procedures
Several techniques, including snip removal, shave removal, hyfrecation, and cryosurgery.
Scissor removal
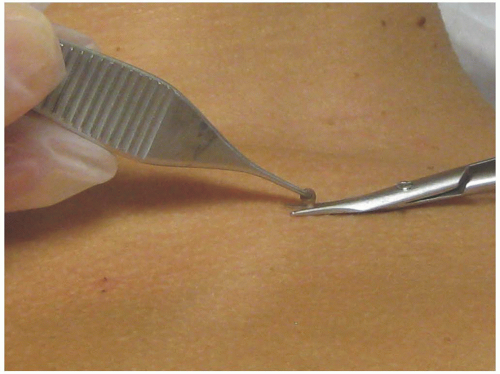
Grasp the skin tag and slightly extend it upward. Make sure to use sharp tissue scissors to assure a quick and accurate snip at the base of the lesion (Figure 24-5). Bleeding should be controlled with pressure, as it is the least likely method to result in scarring. Ammonium chloride (Drysol) is irritating but can be used if the pressure is ineffective. If bleeding continues, electrocautery/hyfrecation may be used after assurance of anesthesia. Before using cautery, remove all alcohol and ammonium chloride (flammable) that may be left on the skin.
Shave removal
Perform the procedure by gently grasping the acrochordon with forceps, slightly extending the lesion upward. Using a DermaBlade or scalpel, shave the lesion at the base (similar
technique as shave biopsy). This is the preferred technique for a larger acrochordon or fibroepithelial polyps. Control bleeding as above.
Hyfrecation
Touch the base of the skin tag with the tip of the hyfrecator (high-frequency eradicator) set at lowest frequency. Contact the skin at a few second intervals until the base turns white/gray. Limit the contacts to three for each skin tag if possible.
Liquid nitrogen
Place the LN2 spray gun nozzle about 1.5 cm from the lesion and aim at the center. Spray until a minimal ice ball encompasses the skin tag. Do up to three freeze and thaw cycles. Freeze times that exceed 30 seconds or are performed at very close range may result in significant tissue damage and hyperpigmentation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree