Fig. 13.1
The PassPort™ patch (a) and the array of filaments (b) on the PassPort™ system
Fig. 13.2
Handheld applicator of the PassPort™ system
The moxibustion therapy is based on the alteration of temperature to influence the skin permeation, absorption, and distribution of the drug in the skin barrier. Moxibustion may have an effect on the local vasodilation response, because it increases the skin temperature. When the body is in warm environment, the vein and capillary are more dilated than that in cold environment, and the blood flow is higher in warm than in cold environment. Permeability of the endothelial membrane of blood vessels and capillaries for drugs becomes higher in warm than in cold environment (Fig. 13.3a). In addition, if inflammation is observed in the tissues, an enhanced permeation retention (EPR) (Fig. 13.3b) effect may be obtained. In other words, tight junctions in the endothelial membrane of blood vessels and capillaries are enlarged in the inflamed condition. Then, moxibustion may increase the skin and muscle concentration of topically applied drugs.
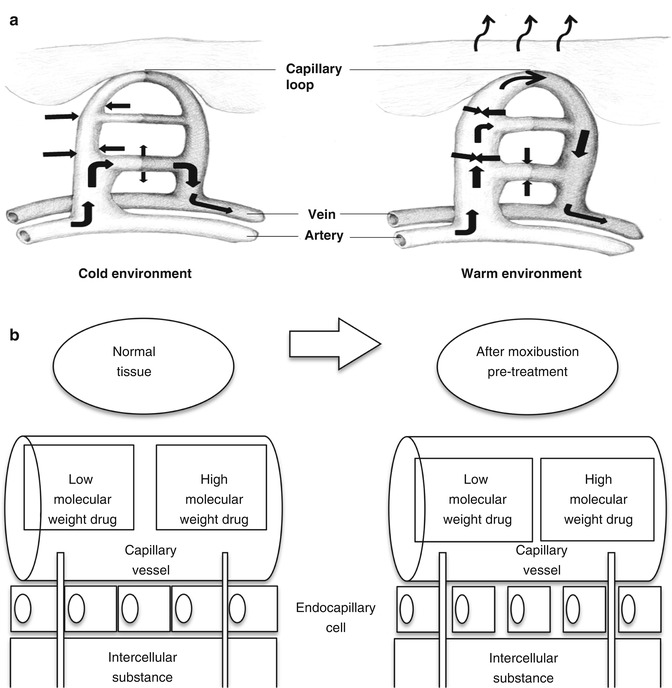

Fig. 13.3
(a, b) Schematic representation of effects of moxibustion on vein and enhanced permeation retention (EPR)
In the present chapter, the in vitro and in vivo pretreatment effects of moxibustion on the skin permeation, as well as on the distribution of an ionic compound, sodium salicylate (SA-Na), in the skin and muscle underneath the application site of moxibustion, are going to be discussed.
13.2 Moxibustion
Moxa is a natural plant which belongs to (Artemesia family) that consists of several natural plants and is known to contain heptatriacontane and tannins having catechol derivatives (Kobayashi 1988). Moxa has been used for a long time as a traditional medicine for its bactericidal and antifungal properties, especially in moxibustion treatment, an oriental traditional physical therapy which involves burning a herb close to the skin by using a moxibustion device. It has also been utilized for muscle pain relief. The treatment has a long history of about 2000 years in China and about 1000 years in Japan. Moxibustion therapy induces medical effects, especially by stimulating acupoints in the skin. Recently, moxibustion has been reevaluated from a pharmacological point of view (Chiba et al. 1997; Uchida et al. 2003). A series of positive pharmacological effects were generated by the intense heat of moxibustion. These may include prevention of disease, activation of body’s immune system, inhibition of cytotoxicity, and other therapeutic actions and promising modalities. Moxibustion induced various inflammatory responses, such as blood vessel reaction and enhancement of microvascular permeability (Okazaki et al. 1990). A clinical diagnosis can be used to determine if the skin was engorged with blood and whether there are skin blisters after moxibustion treatment.
Typical model of a moxibustion device supplied by Senefa Co. (Nagahama, Shiga, Japan) is shown in Fig. 13.4.
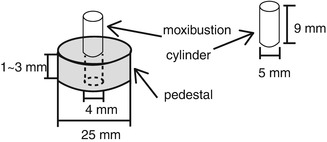

Fig. 13.4
View showing a frame format of moxibustion device which has a moxa cylinder (5-mm diameter × 9-mm length) and a custom pedestal (diameter, 25 mm; thickness, 1–3 mm; hole diameter, 4 mm)
13.3 Skin Temperature
Since the thickness of the pedestal on the moxibustion cylinder may greatly influence the skin temperature as well as the moxibustion therapy, it may also affect the skin permeation of SA after moxibustion pretreatment. Thus, the effect of moxibustion treatment on the temperature change on the skin surface and in the subcutis was evaluated. Figure 13.5 shows the time course of temperature at the skin surface and subcutaneous tissue after moxibustion pretreatment. The maximum skin surface temperature and subcutaneous tissue temperature were 43.7, 45.6, and 47.9 °C and 42.4, 44.7, and 46.5 °C, respectively, for 3-, 2-, and 1-mm pedestal thickness. These temperatures were returned to the control (initial) value (without or before moxibustion) within 10–15 min. The efficacy of heating the skin was higher when using a thinner pedestal; therefore, decreasing the distance between the skin surface and moxa increased the skin temperature. Large variations were found in the skin temperature when using a 1.0-mm pedestal. Figure 13.4 shows the pretreatment technique of topically applied moxibustion on the skin.

Fig. 13.5
View showing the pretreatment effect of topically applied moxibustion on the skin permeation of SA
13.4 In Vitro Skin Application of Na-SA
Sodium salicylate (Na-SA) solution was prepared in order to increase the solubility of salicylic acid (SA); however, only SA concentration was measured after topical or intravenous administration of Na-SA solution in order to evaluate the pretreatment effect of moxibustion on the skin permeation of SA. In vitro permeation experiment was performed to assess the effect of the pedestal thickness of the moxibustion system on the skin permeation of SA. The abdominal skin of hairless rat was treated one time or three times consecutively for 5.0 min with moxibustion. The skin permeation experiment of SA was started 30 min after starting the first moxa burning. The effect of the number of moxibustion cylinders and the distance between the cylinder tip and skin surface on the in vitro skin permeation of SA were evaluated. Figure 13.6 shows the effect of moxibustion pretreatment on the time course of the cumulative amount of SA that permeated through hairless rat skin.
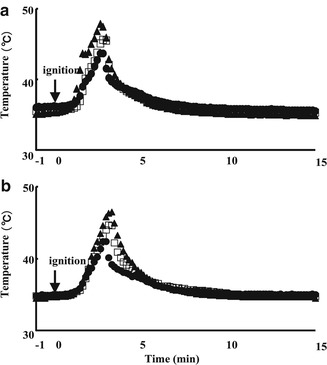

Fig. 13.6
Effect of moxibustion pretreatment on the temperature change on the skin surface (a) and in the subcutis. (b) ●, 3-mm pedestal thickness; □, 2-mm pedestal thickness; ▲, 1-mm pedestal thickness. Each data point represents the mean value
Table 13.1 summarizes the different permeability coefficients obtained for SA for different moxibustion pretreatments.
Table 13.1
Permeability coefficients of SA through excised hairless rat skin
Pretreatment | Permeability coefficient (cm/s) |
|---|---|
Without moxibustion | (2.33 ± 0.007) × 10−8 |
Moxa 1 with a 2-mm pedestal | (4.53 ± 1.47) × 10−8 |
Moxa 3 with a 1-mm pedestal | (7.56 ± 0.008) × 10−8 |
Moxa 3 with a 2-mm pedestal | (1.25 ± 0.002) × 10−7 |
Moxa 3 with a 3-mm pedestal | (3.71 ± 0.005) × 10−7 |
The abdominal skin of hairless rat was treated one time or three times consecutively for 5.0 min with moxibustion. The excised skin was sandwiched between two half-diffusion cells with an effective diffusion area of 0.95 cm2 and a cell volume of 2.5 ml. Next the Na-SA solution was applied on the stratum corneum side of the excised skin for 8 h. The cumulative amount of SA permeated after 8 h was 2.4-, 3.1-, 6.2-, and 21-fold higher than in the control experiment (without moxibustion) for one moxibustion with 2-mm pedestal and three moxa cylinders with 3-, 2-, and 1-mm pedestal, respectively. The thickness of the pedestal of the moxa cylinder was very important for the effect of moxibustion on the skin permeation of SA. Increasing the number of moxa cylinders and decreasing the pedestal thickness increased the skin permeation of SA.
13.5 Intravenous Injection of Na-SA
The abdominal skin of hairless rat was treated three times consecutively for 5.0 min with moxibustion. SA-Na (5 mg/kg) was intravenously injected through the left jugular vein 30 min after starting the first moxibustion, in order to evaluate the skin and muscle disposition of topically applied SA as well as its elimination kinetics. Blood sampling was performed periodically from the right jugular vein. The obtained blood sample was centrifuged at 4 °C to obtain plasma. At the end of the experiment (8 h after the injection of SA-Na), the skin and muscle tissues (2.5 cm diameter under the moxibustion site) were excised, and the tissue samples were kept until analysis. Time course of plasma concentration and obtained pharmacokinetic parameters for SA are shown in Fig. 13.7 and Table 13.2, respectively. No significant difference was observed with and without moxibustion pretreatment in the elimination pharmacokinetics of SA, suggesting that moxibustion did not affect the elimination kinetics from the systemic circulation.