The supraclavicular artery perforator (SAP) flap is a versatile flap for the reconstruction of head and neck defects. Recently, the authors have modified the SAP flap by using an anterior branch of the transverse cervical artery. The anterior SAP flap allows the harvest of a tissue island in the deltopectoral fossa, which is even thinner, is more pliable, and shows a superior color match to the face and neck compared with the original SAP flap. Pre-expansion increases flap size considerably, enabling the coverage of extended defects without the need of microsurgery.
Key points
- •
The pedicled anterior supraclavicular artery perforator (a-SAP) flap is a versatile flap in head and neck reconstruction.
- •
The a-SAP flap uses the anterior branch of the transverse cervical artery.
- •
The skin of the deltopectoral fossa is thinner, more pliable, and shows a better color match compared with the original supraclavicular artery perforator (SAP) flap.
- •
Pre-expansion allows the reconstruction of extended cervical and facial defects.
Introduction
Since its first description in 1997, the supraclavicular island flap (SIF) has emerged as a respected technique for the reconstruction of defects mainly located in the head and neck area, which remain a challenge to plastic and reconstructive surgeons. The advantages of the SIF are manifold and include its minor donor site morbidity, its safety and reliability, and most importantly its favorable match in color and texture to tissue of the head and neck area. Over the years, the SIF underwent several decisive modifications. Although a more difficult procedure, tunneling of the SIF markedly reduces scars at the donor site. In the same vein, the authors proposed a pre-expansion of the donor site, which permits an increase flap size and a considerable thinning of the flap with enhanced microcirculation.
The most recent refinement of the SIF resulted in the renaming of the SIF to the SAP flap (because the supraclavicular artery pierces the platysma and is, therefore, considered a perforator in agreement with the Gent consensus on perforator flap terminology ), and the development of a new flap named the a-SAP flap. The supraclavicular artery, which is the pedicle of the SAP flap, is a branch of the transverse cervical artery. In the a-SAP flap, however, an anterior supraclavicular pedicle that originates separately from the transverse cervical artery serves as the main supplying artery. The anterior supraclavicular artery is as reliable as the supraclavicular artery but allows the harvest of a more anteriorly located flap. In the authors’ department, the a-SAP flap is almost exclusively used due to its undeniable benefits over the SAP flap. This article reviews the surgical technique, complications, and postoperative outcomes of the pre-expanded a-SAP flap based on the current literature and many years of experience in the authors’ department.
Introduction
Since its first description in 1997, the supraclavicular island flap (SIF) has emerged as a respected technique for the reconstruction of defects mainly located in the head and neck area, which remain a challenge to plastic and reconstructive surgeons. The advantages of the SIF are manifold and include its minor donor site morbidity, its safety and reliability, and most importantly its favorable match in color and texture to tissue of the head and neck area. Over the years, the SIF underwent several decisive modifications. Although a more difficult procedure, tunneling of the SIF markedly reduces scars at the donor site. In the same vein, the authors proposed a pre-expansion of the donor site, which permits an increase flap size and a considerable thinning of the flap with enhanced microcirculation.
The most recent refinement of the SIF resulted in the renaming of the SIF to the SAP flap (because the supraclavicular artery pierces the platysma and is, therefore, considered a perforator in agreement with the Gent consensus on perforator flap terminology ), and the development of a new flap named the a-SAP flap. The supraclavicular artery, which is the pedicle of the SAP flap, is a branch of the transverse cervical artery. In the a-SAP flap, however, an anterior supraclavicular pedicle that originates separately from the transverse cervical artery serves as the main supplying artery. The anterior supraclavicular artery is as reliable as the supraclavicular artery but allows the harvest of a more anteriorly located flap. In the authors’ department, the a-SAP flap is almost exclusively used due to its undeniable benefits over the SAP flap. This article reviews the surgical technique, complications, and postoperative outcomes of the pre-expanded a-SAP flap based on the current literature and many years of experience in the authors’ department.
Treatment goals and planned outcomes
As discussed previously, defects that are primarily reconstructed by the a-SAP flap are located in the head and neck area. The a-SAP flap is used to restore and improve defects that are congenital or related to infection, disease, tumors, and trauma, including burn injuries. The a-SAP flap unfolds its full benefits especially in large defects that cannot be satisfactorily covered by primary closure or other local solutions. Pre-expansion of the harvest area markedly increases flap size and allows the reconstruction of extensive facial and cervical defects. In some cases, the SAP and a-SAP flap also can be used as a free flap.
Preoperative planning, preparation, and patient positioning
Defects of substantial size can be covered with a-SAP flaps without prior pre-expansion and primary closure of the donor site. To cover more extended defects, the authors routinely pre-expand the a-SAP flap. The donor site must be free of infection and patients and/or relatives must be well educated about the procedure and care, including pain that is par for the course, to reach high compliance during the arduous pre-expansion process. A sample of the defect may help to approximately estimate the position of the a-SAP flap and the size of the needed expander. Coverage by ipsilateral, contralateral, and even bilateral a-SAP flaps are commonly performed in the authors’ department. The first surgery involves the implantation of a tissue expander above the muscle fascia in the deltopectoral fossa through a small axillary incision. The general concept of the pre-expanded a-SAP flap is shown in Fig. 1 . Both operations, the implantation of the expander and the later defect coverage by the pre-expanded a-SAP flap, are performed in supine position with sufficient padding of the patient. When the defect is directly adjacent to the tissue flap, caution is warranted to prevent expansion of the defect tissue rather than the a-SAP flap. The port is usually placed in the medial part of the upper arm.
Procedural approach
The operative procedure of a standard pre-expanded a-SAP flap is illustrated in Figs. 2–4 in a 5-year-old male patient with extended burn scars in the head and neck area. The authors covered the left buccal area by a pre-expanded ipsilateral a-SAP flap. In Figs. 2 and 3 , the inflated expander and the surgical transposition of the flap are shown. Fig. 4 depicts the transaction procedure of the pedicle.
Once the donor site is sufficiently expanded to cover the recipient site, the second operation can be planned (see Fig. 2 A, B). The a-SAP is a reliable vessel that allows the tissue harvest from a more anterior region than the SAP flap in the deltopectoral fossa (see Fig. 2 C). It pierces the platysma between the origin of the 2 sternocleidomastoid muscle parts, crosses the clavicle in its junction between medial and central third, and runs down to the deltopectoral fossa (dashed line in Fig. 2 C). The artery is preoperatively located by a Doppler probe and marked on the skin. A template is used to determine the exact location and size, and surplus length may be designed to avoid later dog ears.
First, the scar tissue of the recipient site is excised completely (see Fig. 2 D, E). Next, the a-SAP flap is dissected. After skin incision, the expander and optionally its capsule are removed (see Fig. 2 D). The flap is elevated above the muscle fascia from the lateral to the medial portion (see Fig. 3 A), and the anterior branch is detected by intraoperative Doppler or diaphanoscopy (see Fig. 3 B) when dissection reaches the clavicle. The anterior branch is traced to its origin where it takes off the transverse cervical artery. Two veins accompany the anterior branch, of which one drains into the transverse cervical vein and the other into the external jugular vein. When veins and artery are identified and dissected from the surrounding tissue, the a-SAP flap can be rotated into the recipient site (see Fig. 3 C, D).
Final fixation of the flap is performed in a double-layered fashion with subcutaneous absorbable sutures and cutaneous sutures with nonabsorbable material. Wound drains are placed and removed in the early postoperative care; the donor site is closed primarily (see Fig. 3 E).
The authors often perform the a-SAP in 2 surgical steps. In the first step, the a-SAP is transposed to its recipient site and the pedicle is not completely sutured. Instead, the pedicle is covered by a skin substitute (eg, Mepilex AG; Mölnycke Health Care, Düsseldorf, Germany) and transected after approximately 14 days when the a-SAP flap is perfused by the surrounding tissue of the recipient site. In Fig. 4 , the surgical step of pedicle transection is illustrated. Transection of the pedicle is only possible when clamping of the pedicle does not significantly alter flap perfusion (see Fig. 4 A). The flap is then disconnected and the blood vessels ligated (see Fig. 4 B). The a-SAP flap is now perfused by its adjacent tissue. The flap is trimmed and superfluous tissue of the pedicle can be used to cover additional defects (see Fig. 4 C, D).
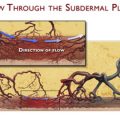
In cases of distant localization of the recipient site from the donor site with significant amount of healthy tissue in between, the a-SAP flap also can be tunneled, which reduces visible scars in the neck area. After complete incision of the flap, the pedicle with its surrounding fatty tissue is first dissected on the epifascial level and then in the subdermal layer. Finally, the a-SAP flap is gently pulled through a subcutaneous tunnel to its recipient site.
Under special circumstances, the a-SAP flap can be used as an osteocutaneous flap. When the incision reaches the periosteum, a bone chip is harvested from the lateral end of the clavicle or the acromion. The size of the bone chip may vary and should be adapted to the defect (eg, the cartilaginous defect of the trachea after decannulation). The a-SAP flap is then transposed to the tracheal defect and the bone chip is secured with slowly absorbable sutures.
The a-SAP flap also can be transferred as a free flap. Flap dissection does not differ from the usual preparation of the pedicled a-SAP flap. The artery is followed proximally to the transverse cervical artery and transected when artery diameter reaches approximately 1.5 mm. After transection of the vein that drains into the external jugular vein, the flap can be anastomosed at the recipient site either end-do-end or end-to-side.
Potential complications and management
Complications of the pre-expanded a-SAP flap can be divided into those related to the pre-expansion procedure and those that are caused by the explicit a-SAP flap transposition.
First, careful patient selection is the most important measure to avoid unfavorable results or complications during the tissue expansion. Those include hematomas, seromas, the dislocation, deflation, or exposure of the expander/port, infection, and necrosis. Minor hematomas and seromas may be aspirated preferably under ultrasound control to avoid damage to the expander and mostly resolve without surgical intervention. In other cases, however, a surgical evacuation of the hematoma and seroma, repositioning or replacement of dislocated/deflated expanders/ports, or even removal of the expander to manage infections and necrosis is necessary. To prevent infection and hematoma, meticulous intraoperative hemostasis and strict antiseptic precautions during the surgery and later inflation of the expander are pivotal. Cunha and colleagues evaluated 315 tissue expanders in a total of 164 patients and found an average 22.2% of complications. The highest complication rates were found in the face and neck area and the scalp whereas the trunk showed the lowest. This report is in line with the authors’ experience, rarely observing major complications of the tissue expansion in the deltopectoral fossa.
Although the vascularization of the a-SAP flap is reliable with a consistent artery and 2 veins, perfusion depending on the flap size is the most critical aspect. In cases of extended size of the flap or the iatrogenic damage of the pedicle during flap dissection, a delay procedure appears to be a potential remedy. Although the a-SAP is the main blood supplier of the flap, the flap also is party perfused via the surrounding tissue. During the delay procedure, the a-SAP flap is incised at its borders enabling the pedicle to assume its role as the main circulation source. In a second delayed procedure, the flap is raised and transposed to its proper location.
Other general complications may be considered, particularly in early postprocedural care, including hematomas, infections, wound healing disorders, donor site morbidity, and so forth, that may be managed with appropriate standardized measures. In larger studies involving more than 20 cases, a major complication rate of less than 5% and minor complication rate of approximately 10% for the SAP flap was reported. Herr and colleagues investigated the impact of the supraclavicular artery island flaps on shoulder function in 10 patients and found some limitation in the range of motion whereas the strength and function were not restricted.
Postprocedural care, rehabilitation, and recovery
The implantation of the tissue expander can be done as an outpatient operation. After 2 to 3 weeks of uneventful primary wound healing, the expander is perpetually filled through the port, which is normally placed at the medial upper arm where it is easily palpable. The inflation process is routinely performed as an office procedure on a weekly basis until the necessary expansion volume is reached. After a period of approximately 2 weeks after the final inflation, the surgical flap transposition is carried out, usually as an inpatient surgery. The postprocedural care does not vary markedly from other local flap surgeries. In the first days after surgery, disturbances of flap perfusion by mechanical stress should be avoided by adequate positioning of the arm and limited bed rest.
Outcomes
Case 1
Fig. 5 shows the preoperative and postoperative pictures of the 5-year-old patient with disfiguring facial burn scars who was treated by a ipsilateral pre-expanded a-SAP flap. The postoperative pictures taken 4 years after surgery show the good color match and the excellent reconstruction of the facial contour. The scars on the scalp were not reconstructed yet due to the soft skull bone and reconstruction is planned after completion of growth.