Guidelines for appropriate use of superficial radiation therapy are based on decades of research; although no formal appropriate use criteria have been developed, they are warranted. Superficial radiation in the outpatient dermatologic setting is the least expensive form of radiation treatment. Although higher cure rates may be possible with Mohs surgery, this should never argue against dermatologists retaining and refining a modality, nor should we limit its use by our successors. Most important, our elderly and infirm patients should continue to benefit from superficial radiation therapy in outpatient dermatologic settings.
Key points
- •
Superficial radiation therapy has more than 106 years of research and development by dermatologists.
- •
Compared with hospital-based radiation therapy delivered by radiation oncologists, superficial radiation delivered in the outpatient dermatologic setting is the least expensive form of radiation treatment.
- •
Superficial radiation therapy is currently an underused modality in the treatment of nonmelanoma skin cancer.
- •
With the aging, feeble population it is vital to keep this cost-efficient modality within the hands of dermatologists.
Superficial radiation therapy (SRT) has been the standard of care for office-based radiation treatment of nonmelanoma skin cancers (NMSC) for more than 100 years. This began with Leopold Freund (acknowledged as the father of radiation therapy [RT]), with a continuous lineage from him to Pusey, MacKee, Cippolaro, Goldsmith, Gladstein, Panizzon, and Kopf. SRT was an integral part of dermatology practices for the better part of the 20th century until the mid 1980s, when the popularity of Mohs surgery and the cessation of manufacturing of new and modern SRT platforms led to a decrease in the popularity of SRT. An American Academy of Dermatology (AAD) task force in 1974 concluded that 55% of dermatology offices used SRT and 44% of dermatologists used SRT regularly.
The physics of SRT are less complex than any laser/light platform in use by dermatologists today. X-rays are part of the electromagnetic spectrum beginning just beyond the ultraviolet spectrum. Dermatologists have embraced and pioneered use of the entire electromagnetic spectrum. From conception, design, and fine tuning, to use, SRT remains within the purview of dermatologic therapy. Dermatologists consider themselves cutaneous oncologists, using imaging, targeted therapy, immunotherapy, surgery, and primary as well as adjunctive radiotherapy to treat skin cancer. With the aging, feeble population it is vital to keep this cost-efficient modality within the hands of dermatologists.
Dermatology has played a pivotal role in the conception and development of many other subspecialties such as rheumatology, venereology, cosmetic surgery, and radiation oncology. Long before the American Club of Therapeutic Radiologists was formed in 1962, (the precursor to the American Society for Therapeutic Radiology and Oncology [ASTRO]), dermatologists were pioneering the use of RT and brachytherapy. A de facto relinquishment of SRT to radiation oncologists would be comparable with (1) relinquishment of laser therapy to plastic surgeons because it falls within the realm of cosmetic treatment, (2) Mohs surgery to general and plastic surgeons simply because it is surgery, or (3) dermatopathology to general pathologists.
Best practices in dermatologic radiotherapy
Best practice includes consensus development based on best evidence formulated by dermatologists with the most experience using a particular radiation modality. This practice includes the creation of a treatment algorithm for NMSC and incorporation of SRT into the treatment algorithm for the population over the age of 65. At present, guidelines exist based on past research, though no appropriate use criteria (AUC) have been developed. The most critical aspect of SRT use is appropriate patient and tumor selection.
The following are proposed SRT AUC for basal cell carcinoma (BCC)/squamous cell carcinoma (SCC) treatment that are accepted by experienced dermatology radiotherapists in the past and present:
- 1.
Location: central face, including the eyelids, nasal tip, nasal ala, ears, lips.
- 2.
Age ≥60 years: to minimize the synergistic effects of ultraviolet radiation and late sequelae.
- 3.
Tumor size: tumors up to 5 cm in diameter may be adequately treated with SRT.
- 4.
Tumor type/depth of invasion: superficial and nodular BCCs, SCC in situ, and SCC that are nonaggressive are amenable to SRT.
- 5.
Frailty and medical status: inability to tolerate surgery owing to poor health, multiple comorbidities, or those on anticoagulant therapy may have a higher risk of adverse surgical events. Eastern Cooperative Oncology Group performance status may be used to document selection of radiotherapy over surgery.
- 6.
Patient preference to avoid surgery may be a consideration and in cases where surgery will lead to skin graft or complex flap closure.
Absolute (1–4) and relative (5,6) contraindications for SRT include the following:
- 1.
Aggressive tumor histology: BCCs (sclerosing, morpheaform, infiltrative), SCC (perineural invasion, arising in previous sites of RT, burn scars, chronic ulcers, spindle cell carcinoma, poorly/undifferentiated, or those secondary to osteomyelitis).
- 2.
Deep tumor invasion: tumors that invade bone, cartilage, or arise within the mucosal surfaces (intranasal/intraoral).
- 3.
Previously irradiated site: increases incidence of late-term sequelae (ulcer, radionecrosis of cartilage and bone) results in unsatisfactory cosmesis, recurrence, and second primary tumors.
- 4.
Genetic anomalies: nevoid BCC syndrome, xeroderma pigmentosum, Garner’s syndrome, Li–Fraumeni syndrome, and others with increased radiosensitivity or where radiation may induce new malignancies.
- 5.
Organ transplant recipients: the mainstay of treatment is surgical excision or Mohs surgery.
- 6.
Location on the trunk or extremities: early pioneers of SRT recommended against the use of radiotherapy on the trunk and extremities owing to late sequelae changes (telengiectasias and pigmentary changes), lower oxygen saturation leading to potential decreased efficacy and wound healing issues, and the general ease and expediency of surgical removal.
Best practices in dermatologic radiotherapy
Best practice includes consensus development based on best evidence formulated by dermatologists with the most experience using a particular radiation modality. This practice includes the creation of a treatment algorithm for NMSC and incorporation of SRT into the treatment algorithm for the population over the age of 65. At present, guidelines exist based on past research, though no appropriate use criteria (AUC) have been developed. The most critical aspect of SRT use is appropriate patient and tumor selection.
The following are proposed SRT AUC for basal cell carcinoma (BCC)/squamous cell carcinoma (SCC) treatment that are accepted by experienced dermatology radiotherapists in the past and present:
- 1.
Location: central face, including the eyelids, nasal tip, nasal ala, ears, lips.
- 2.
Age ≥60 years: to minimize the synergistic effects of ultraviolet radiation and late sequelae.
- 3.
Tumor size: tumors up to 5 cm in diameter may be adequately treated with SRT.
- 4.
Tumor type/depth of invasion: superficial and nodular BCCs, SCC in situ, and SCC that are nonaggressive are amenable to SRT.
- 5.
Frailty and medical status: inability to tolerate surgery owing to poor health, multiple comorbidities, or those on anticoagulant therapy may have a higher risk of adverse surgical events. Eastern Cooperative Oncology Group performance status may be used to document selection of radiotherapy over surgery.
- 6.
Patient preference to avoid surgery may be a consideration and in cases where surgery will lead to skin graft or complex flap closure.
Absolute (1–4) and relative (5,6) contraindications for SRT include the following:
- 1.
Aggressive tumor histology: BCCs (sclerosing, morpheaform, infiltrative), SCC (perineural invasion, arising in previous sites of RT, burn scars, chronic ulcers, spindle cell carcinoma, poorly/undifferentiated, or those secondary to osteomyelitis).
- 2.
Deep tumor invasion: tumors that invade bone, cartilage, or arise within the mucosal surfaces (intranasal/intraoral).
- 3.
Previously irradiated site: increases incidence of late-term sequelae (ulcer, radionecrosis of cartilage and bone) results in unsatisfactory cosmesis, recurrence, and second primary tumors.
- 4.
Genetic anomalies: nevoid BCC syndrome, xeroderma pigmentosum, Garner’s syndrome, Li–Fraumeni syndrome, and others with increased radiosensitivity or where radiation may induce new malignancies.
- 5.
Organ transplant recipients: the mainstay of treatment is surgical excision or Mohs surgery.
- 6.
Location on the trunk or extremities: early pioneers of SRT recommended against the use of radiotherapy on the trunk and extremities owing to late sequelae changes (telengiectasias and pigmentary changes), lower oxygen saturation leading to potential decreased efficacy and wound healing issues, and the general ease and expediency of surgical removal.
Estimate of current practice
The 1974 AAD Task Force on Ionizing Radiation conducted a comprehensive survey sending a detailed questionnaire to 4560 dermatologists in the United States and Canada. Of the 2444 replies, 44% of respondents (1075) reported using radiotherapy weekly. Superficial x-ray or Grenz-ray equipment was reported to be available in 55.5% of dermatologic offices. A larger pool of dermatologists in the past had considerable experience with the use of in-office radiotherapy and helped to shape, by their use and research, the guidelines in use today. Recently, there has been a noticeable resurgence in the interest and use of SRT by dermatologists with the reintroduction of more modern, user-friendly, and safer equipment. Research is currently being undertaken to ascertain current SRT utilization rates.
Practice gaps in the use of superficial radiation therapy in clinical dermatology practice
Knowledge Gap Within Dermatology Professional Organizations
In a 2013 position statement, the AAD grouped newer technologies such as high-dose electronic brachytherapy (EBT), together with SRT as a “new” therapy with the need for “research on long-term outcomes.” The AAD has since clarified these modalities in an addendum position statement. Nonetheless, the AAD continues to mistakenly refer to SRT as a “new” technology that “differs substantially from traditional external beam radiation therapy” and one in need of “research on long term outcomes.” This is despite the fact that SRT was the standard radiation modality in use by dermatologists long before electron beam therapy was conceived or used. Goldschmidt in his 1978 book “Physical Modalities in Dermatologic Therapy” reports that superficial x-ray machines were the most commonly used dermatologic radiotherapy units in the United States and Canada in 1975. Similarly, long-term SRT outcomes have been reported in the past and continue to be reported by dermatologists.
Perceived Research Gaps
SRT has more than a century’s worth of research and development. Dermatologists were the first radiation oncologists applying SRT to treat skin disease as early as 1897, shortly after the discovery of x-rays by Wilhelm C. Roentgen in 1895, and decades before the specialties of radiology and radiation oncology emerged and then diverged. Pioneers in radiotherapy, dermatologists authored, and more recently coauthored with radiation oncologists, textbooks that have been in continuous publication since 1921 on the use of SRT and radium in skin disease. The first reported results using radiotherapy came from dermatologists in what was the precursor to the annual AAD meeting, “Rationale of and the Indications for Therapeutic Use of Rontgen Rays” (27th Annual Meeting of the American Dermatological Association, Washington, May 13th and 14th, 1903).
Dermatologists were the first to look at fractionation and its effects on early and late sequelae. Dermatologists formulated fractionation schemes and time dose fractionation formulas enabling treatment plan optimization. They were the first to incorporate the D 1/2 concept to ensure adequate depth radiation dose to the tumor base. The body of knowledge and experience that we have inherited from our predecessors in dermatologic radiotherapy is immense and still valid today.
The Randomized, Clinical Trials Conundrum Gap
The AAD in their recent Position Statement on SRT and Electronic Brachytherapy for BCC and SCC disallowed all studies that were not randomized, clinical trials (RCTs). Of note, authors Levy and Stasko in Alams’ book “Evidenced-Based Procedural Dermatology” uncovered only 2 RCTs involving Mohs micrographic surgery, both comparing Mohs micrographic surgery with surgical excision. They state that the bulk of literature regarding Mohs micrographic surgery consists of case studies, systematic reviews, and meta-analyses. They note that most of the literature on Mohs micrographic surgery is retrospective, nonrandomized, and suffers from selection bias. This is the same argument used by most critics of SRT, yet Mohs micrographic surgery is still accepted as the treatment of choice for NMSC in many situations.
The authors report that “the level of risk for a type of skin cancer often drives the decision of which treatment is used.” It is on this basis, as well as patient factors, that SRT is offered to select patients/tumors. Similar to Mohs micrographic surgery, the vast majority of research that has formed the foundation of dermatologic radiotherapy has come from retrospective analysis of patients chosen for SRT. From these studies we have gained an in- depth understanding of optimal dosimetry based on tumor characteristics and patient fact ors.
Randomized trials for the treatment of skin cancer are near impossible to undertake. To assign patients randomly to various modalities today would increase significantly the risk of recurrence, morbidity, and even mortality. For example, megavoltage RT is often used as a primary treatment or adjunct to surgery for tumors invading bone and orbital structures; however, one would never randomize a cohort of patients with carcinoma invading bone to Mohs surgery versus treatment with RT alone.
An additional argument against SRT is that studies involving RT should confirm the long-term clearance of residual tumor based on histologic analysis of the irradiated tissue several years later. This unnecessary step, in light of clinical follow-up, further subjects the patient to increased morbidity by excision or multiple random biopsies. We could uncover no studies that follow NMSC surgery patients for 5 years then re-excise the scar created from the initial surgery to histologically confirm the absence of residual tumor. The expectation that this should be done with RT is equally implausible.
National Comprehensive Cancer Network Guidelines Gap
The National Comprehensive Cancer Network (NCCN) established guidelines for the treatment of NMSC to include RT; however, only electron beam RT and orthovoltage RT, performed by radiation oncologists, are included in the consensus guidelines. These guidelines fail to mention SRT as a modality to treat NMSC. Of the 28 panel members that developed the NMSC NCCN guidelines, 16 are dermatologists (10 Mohs surgeons), 5 are medical oncologists, 5 are surgical oncologists, 1 is a pathology trained dermatopathologist, and 1 is a radiation oncologist.
Use Gap
In 1981, Goldschmidt noted that RT had been used routinely in the treatment of 10% to 20% of skin cancers in leading dermatologic institutions where all other forms of therapy were also available. Goldschmidt noted that 400,000 new cases of skin cancer were occurring each year in the United States (1981); if dermatologists were to cease using ionizing radiation, 40,000 to 80,000 patients would have to be treated with other modalities that may not have been the treatment of choice, such as surgery or more expensive RT modalities ( Table 1 ).
| Treatment Method | Total Cost to Treat Including Recurrences |
|---|---|
| Mohs micrographic surgery: nose/eyelid with FTSG repair | 1767.50 $US |
| Mohs micrographic surgery: nose/eyelid with flap repair | 1670.60 $US |
| Dermatologic office-based radiation (5 fractions) used in our practice | 687.38 $US |
| Dermatologic office-based superficial radiation (12 fractions) | 1019.20 $US |
| High-dose rate electronic brachytherapy (8 fractions) | 8046.86 $US |
| Hospital-based orthovoltage radiation (20 fractions) | 3889.80 $US |
| Hospital-based megavoltage radiation/electron beam (20 fractions) | 7281.79 $US |
The senior author estimates that 500 SRT units are in use across the United States. With about 10,000 actively practicing dermatologists, 1 out of 20 dermatologists (5%) are offering this modality as a treatment option. Based on our own experience, 5% of patients diagnosed with, or referred for, NMSC treatment are eligible for and choose treatment with SRT. Extrapolated this represents a 0.25% overall use of SRT by dermatologists, whereas 5% is likely an optimal use rate.
We surveyed a random sample of dermatologists practicing in the United States regarding their use of SRT for the treatment of skin cancer. Of 67 respondents, 60 (90%) stated they did not use radiotherapy in their office on a weekly basis and 58 of the 67 (87%) did not have radiation equipment available. Only 15 of the 67 (22%) reported an SRT use rate of 1% to 5% for BCC treatment and 50 of the 67 (75%) reported no use at all for BCC. Only 13 (19%) reported an SRT utilization rate of 1% to 5% for SCC treatment and 53 (79%) reported no use for SCC. These responses are in line with our hypothesis that SRT is underused in the treatment of NMSC. Research is currently being undertaken to ascertain rates of use from Medicare data.
Radiation Therapy Terminology Knowledge Gap
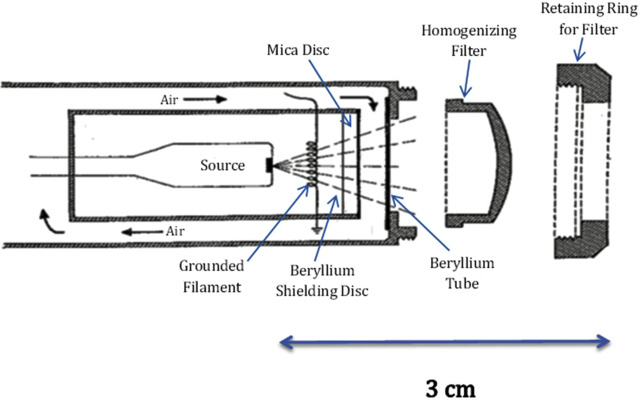
The AAD Position Statement on SRT and EBT for BCC and SCC exemplifies the confusion that exists between various x-ray platforms. The original intent of EBT manufacturers was to provide intracavitary placement of miniaturized cathodes deep in tumor beds for neoplasms such as breast cancer where the delivery of high-dose photons is done only once before surgical closure of the wound. EBT systems use no radioactive gamma-emitting isotopes; they use a conventional but miniaturized x-ray tube that emits a multispectral beam of photon radiation. With a source to surface distance that ranges between 2.5 and 6 cm, EBT may be characterized more appropriately as contact therapy radiation or ultrashort distance radiotherapy. Early predecessors of these x-ray machines (Chaoul and Philips units), with a source to surface distance of 1.5 to 3.0 cm, were in existence in the late 1940s and are still in use today ( Figs. 1 and 2 ).
Today, the term SRT is still used, although modern units emit soft x-rays. Compared with the older superficial x-ray units, they are safer, simpler, and more reliable ( Table 2 ).
| Type | Sources and Synonyms | Type of Generator | kV | SSD (cm) | D 1/2 mm Tissue | Surface Dose (%) a |
|---|---|---|---|---|---|---|
| Megavoltage electron therapy | Electron beam radiation | LINAC | >1000 (6000–9000) | 80 | 90% isodose method used for electrons b | 78–86 |
| Megavoltage photon therapy (not routinely used to treat NMSC) | Megavoltage X-ray | LINAC, Betatron | >1000 | 80 | 150–200 | 6–30 |
| Supervoltage therapy | γ-ray | Isotope teletherapy Machines (60Cobalt) | 400–800 | 50–80 | 80–110 | 40–90 |
| Orthovoltage therapy | Deep x-ray | X-ray machine cathode | 200–400 | 50–80 | 50–80 | 100 |
| Intermediate therapy | Half-deep therapy | X-ray machine cathode | 110–130 | 30 | 30 | 100 |
| Contact therapy | Ultrashort distance (Chaoul) | X-ray machine cathode | 50–60 | 1.5–3.0 | 4–30 | 100 |
| Electronic brachytherapy c | Misnomer when used to treat skin cancer | Miniaturized cathode | 50 | 2.5–6.0 | 3–7 (varies with probe position) | 100 |
| Superficial x-ray therapy d | Pyrex (glass) Window (older units), | X-ray machine cathode | 60–100 | 15–30 | 7–20 | 100 |
| Soft x-ray therapy d | Beryllium Window (modern units) | X-ray machine cathode | 20–100 | 10–30 | 1–20 | 100 |
| Grenz therapy | Ultrasoft therapy, supersoft therapy | X-ray machine cathode | 5–20 | 10–15 | 0.2–0.8 | 100 |
a Surface dose is the percent of radiation dose delivered to the skin surface.
b The 90% Isodose method is used by radiation oncologists for electron beam radiotherapy.
c Depth dose varies with position of the x-ray probe that is used.
d Superficial/soft x-ray therapy is the type most often used in dermatology office-based radiotherapy for squamous cell carcinoma, squamous cell carcinoma in situ, and basal cell carcinoma.
Perceived Expense of Dermatology Office-based Radiotherapy Gap
Prior cost comparisons of RT in dermatologic literature did not differentiate between dermatologic office-based radiotherapy and radiation delivered by a radiation oncologist in a hospital setting. Rogers and Coldiron report the cost of RT for a BCC on the cheek to be $2591 to $3460. Table 3 presents a cost comparison of all RT modalities currently in use today for the treatment of an NMSC. We have calculated the costs to treat a T1-2N0M0 NMSC on the nose or eyelid, 2 locations SRT finds its greatest utility. The cost of Mohs micrographic surgery is calculated using 2 stages of Mohs micrographic surgery and a flap or graft repair (primary closure is often not possible on the nose or eyelid). The number of stages is derived from Alam and colleagues, who reported the number of stages by anatomic location and geographic region for Mohs surgeons across the United States. The average number of stages was 1.96 for the periorbital region and 2.01 for the nose. The multiple surgery reduction rule was applied.
| Need for | More, n (%) | Same, n (%) | Less, n (%) | None, n (%) |
|---|---|---|---|---|
| Practical instruction | 59 (75.64) | 9 (11.54) | 2 (2.56) | 8 (10.26) |
| Theoretic instruction—indications | 60 (76.92) | 9 (11.54) | 2 (2.56) | 7 (8.97) |
| Theoretic instruction—techniques | 56 (71.79) | 11 (14.10) | 3 (3.85) | 8 (10.26) |
| Theoretic instruction—radiation physics | 52 (66.67) | 12 (15.38) | 2 (2.56) | 12 (15.38) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




