Posterior Interosseous Artery Flap
Kate Elzinga
Kevin C. Chung
DEFINITION
The posterior interosseous artery (PIA) flap is a perforator flap used for coverage of upper extremity defects. Its harvest avoids the sacrifice of a major artery, such as that of the radial artery during harvest of the radial artery flap.
It is available in patients without an intact palmar arterial arch.
The PIA flap is typically used as a pedicled flap. It can also be harvested as a free flap.1
The PIA flap is most commonly used as a retrograde regional flap based on the anteroposterior interosseous artery system to cover defects of the first web space, dorsal wrist, dorsal and volar hand, and dorsal thumb up to the proximal interphalangeal joint.
The PIA flap can also be used as an antegrade flap for defects of the elbow, antecubital fossa, and proximal volar forearm.
The dimensions of the PIA flap are designed based on the defect size.
One case series of 53 patients, from whom two-thirds of the circumference of the forearm skin was harvested, reported the use of PIA flaps from 5 × 2.5 cm to 21 × 10 cm in size.2
The PIA flap provides a good color match for defects of the forearm, wrist, and hand, particularly dorsally.
In dark-skinned patients, resurfacing of the palmar skin with a PIA flap leads to a color mismatch.
ANATOMY
The PIA flap is classified as a Mathes and Nahai type B flap; it is supplied by a septocutaneous perforator.
Most commonly, the flap is harvested subfascially as a fasciocutaneous island flap.
It can also be harvested with a skin bridge or as an adipofascial flap, which permits primary closure of the donor site; the subcutaneous tissue and fascia are harvested; the dermis is preserved at the donor site.3
When used as an adipofascial flap, the flap is covered with a split-thickness skin graft (STSG) after inset.
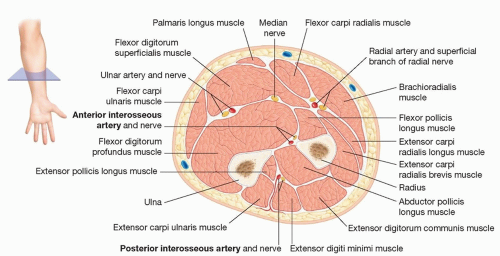
The flap pedicle is composed of the PIA and its two adjacent vena comitantes.
Proximally, the artery is 1.2 to 2.1 mm in diameter and the vena comitantes are 1.0 mm.
Over the middle third of the forearm, the PIA has its narrowest caliber, measuring 0.5 mm.
Distally, it measures 0.9 to 1.1 mm.4
The PIA arises 4 cm from the lateral epicondyle from the common interosseous artery in 80% of cases and directly from the ulnar artery in 20% of cases.5
The anterior interosseous artery (AIA) also arises from the common interosseous artery, or less commonly, directly from the ulnar artery. It runs along the volar surface of the interosseous membrane (IOM), deep to the flexor digitorum profundus and flexor pollicis longus.
The PIA passes through the IOM from the volar forearm to the dorsal forearm 8 cm distal to the lateral epicondyle and 14.5 cm proximal to the ulnar styloid.5
In most cases, it gives origin to three fasciocutaneous perforators to the overlying skin in the proximal forearm and six to eight perforators in the distal forearm.4
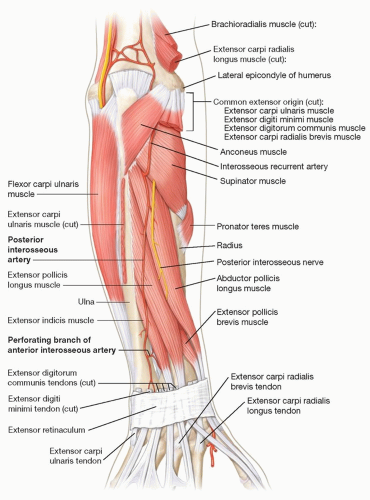
The PIA runs with the posterior interosseous nerve (PIN) along the dorsal IOM to the hand in the septum between the extensor digiti minimi (EDM) and extensor carpi ulnaris (ECU).
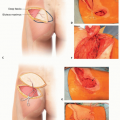
After passing under the supinator in the proximal forearm, the PIA runs over the abductor pollicis longus, extensor pollicis longus, extensor pollicis brevis, and extensor indicis proprius (FIG 1).
Two centimeters proximal to the distal radioulnar joint (DRUJ), just proximal and radial to the head of the ulna, a branch of the PIA travels volarly through the IOM and anastomoses with the dorsal branch of the AIA.
This anastomotic connection is the basis for the retrograde PIA flap.
The PIA continues distally and joins the dorsal carpal arch. The retrograde PIA flap also receives some flow through this arch.
The axis of the flap is marked from the lateral epicondyle of the elbow to the head of the ulna.5
The flap design is centered on the septum between the EDM and ECU to maximize the number of perforators entering the flap from the PIA between the dorsal margins of the ulna and radius (FIG 2).
The flap is typically designed up to 18 × 8 cm in size.
A segment of the proximal third of the ulna, with a cuff of the extensor pollicis longus, can be included in the PIA flap for use as an osteocutaneous flap.6
PATIENT HISTORY AND PHYSICAL FINDINGS
Important aspects of the patient history include age, sex, handedness, occupation and avocations, past medical history, medications, allergies, smoking and recreational drug use, and previous injury to the affected arm.
The wound is evaluated for
Exposed and damaged structures—skin, tendons, neurovascular structures, bone
Health of remaining structures—tissue necrosis, contamination, signs of infection
Local and regional donor sites
Radiographic imaging is used in conjunction with physical examination to determine the presence and extent of bone injuries.
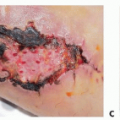
Skin and soft tissue defects can be adequately reconstructed using soft tissue flaps.
When the defect size is too large for local flap coverage, the PIA flap is a useful regional flap that can be performed. It alleviates the need for a microsurgical free flap from a distant donor site.
Many tendon and bony defects can be reconstructed in the hand and then covered with a PIA flap during the same operation. This fasciocutaneous flap provides reliable, durable soft tissue coverage of exposed structures and a smooth gliding surface for underlying tendons.
Skin laxity is assessed over the dorsal forearm using a pinch test.
Flaps larger than 4 to 6 cm in width typically require donor site closure with a skin graft.
If desired, the skin graft can be excised later using serial excision; the patient must be appropriately counseled prior to flap reconstruction regarding the expected appearance of the donor site given its visible location over the dorsal forearm.
IMAGING
Radiographs are performed of the injured extremity to assess for fractures or foreign bodies.
Angiography is not routinely performed prior to PIA flap reconstruction.
If there is concern of the integrity of the PIA or AIA-PIA anastomotic arch, angiography can be considered to evaluate the vessels or a different flap can be selected.
NONOPERATIVE MANAGEMENT
The wound must be adequately debrided and cleansed prior to flap reconstruction. Following traumatic injury, the wound is explored, injured structures are assessed, and necrotic tissue is debrided. Once the wound is clean and the tissues have demarcated, flap reconstruction can proceed.
For the reconstruction of oncologic defects, final tumor pathology and negative margins are established prior to flap closure.
Moist, antibacterial dressings can be used in the interim to minimize tissue desiccation and bacterial load.
SURGICAL MANAGEMENT
Local flaps are limited to coverage of small defects of the upper extremity.
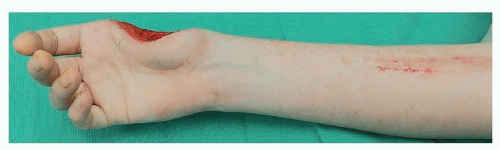
Larger regional flaps, such as the PIA flap, provide coverage of forearm, wrist, and hand defects without the need for free flap microsurgical reconstruction (FIG 3).
Compared with free flaps, pedicled flaps require less general anesthesia time, avoid the need for flap harvest from an uninjured limb or the trunk, and are options when microsurgical equipment or expertise is unavailable.
Preoperative Planning
The risks and benefits of flap reconstruction with a PIA flap are reviewed with the patient.
The benefits include provision of durable, supple soft tissue coverage, a donor site limited to the affected upper extremity, and a single-stage procedure.
The risks include partial or complete flap failure and injury to the PIN. The patient is counseled that the PIA flap has a visible donor site.
When used as a retrograde flap, the PIA flap is insensate.
For antegrade or free PIA flaps, the posterior antebrachial cutaneous nerve of the forearm can be included for sensory restoration.
Previous injury to the PIA flap donor site is a contraindication to its use.
The PIA perforators are small and thin-walled, so crush or shear injury to dorsal forearm makes flap harvest unreliable.
Injury to the DRUJ and possible damage to the AIA-PIA anastomosis precludes retrograde PIA flap use.
Some authors have reported the absence of the AIA-PIA anastomosis or a hypoplastic or absent PIA in the middle third of the forearm. Penteado reported 5 such cases in 110 cadavers, Angrigiani reported 1 case in 80 patients, and Buchler reported 2 cases in 36 patients.7,8,9
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree