Fig. 5.1
The pathophysiological factors and the classical natural history of atopic dermatitis for a patient developing an atopic march (Adapted from Bieber [1])
5.2.1 Environmental Factors
A number of environmental factors have been analyzed in many epidemiological studies over the last three decades. Besides the exposition to highly variable factors like hard water or soaps [29–32] which may directly impact or worsen the well-accepted impaired epidermal barrier function, a number of more specific factors have been highlighted and which may play a crucial role in the sensitization phenomenon as well as in inducing skin inflammation.
5.2.1.1 Allergens
Although the exposition to allergens has been assumed during pregnancy [33–36] and conditioned by the mother’s diet, the most evident exposition to allergen occurs directly after birth. Hereby, food allergens as well as environmental allergens (such as animal dander or pollens) are certainly to be considered with regard to the sensitization phenomenon [33, 35, 37]. However, there is more and more evidence that the disease starts initially without any kind of sensitization and that the latter is most probably started or enforced during the chronic course of the disease in early childhood [2, 38, 39]. This also means that the disease most probably starts as a nonallergic condition where the impaired epidermal barrier function plays a primary role in the induction of the inflammation. However, it is still not clear how genetic mutations or variants of relevant genes for the epidermal barrier function can solely lead to the underlying inflammatory condition. On the other hand, it has been speculated that the contact of foodstuff with the inflamed skin on the perioral region could contribute to the sensitization to food allergens, while the contact of food with the oral mucosa and the gastrointestinal tract would rather lead to tolerance mechanism [40]. This is even supported by recent epidemiological data strongly suggesting that an early introduction of food diversity may be protective against atopic dermatitis [35, 41, 42]. However, it is not clear which individuals may have greater benefit from this kind of strategy.
5.2.1.2 Microbiome and Infections
The role of Staphylococcus aureus and the products thereof has been supported by a number of in vitro and only few in vivo experiments [43, 44]. It is well accepted that the skin of patients with atopic dermatitis is heavily colonized with this bacterium and that the superantigens produced by staphylococci could amplify the inflammatory condition in an allergen-independent way [45, 46]. However, the use of antibiotics and antiseptics seems to only have a limited efficacy in controlling the flares of atopic dermatitis [47–49]. The overall role of bacteria on the skin in atopic dermatitis has more recently been highlighted by new insights in the diversity and complexity of the skin microbiome [50, 51]. Indeed, it appears that healthy skin is characterized by the colonization of at least 250–500 different bacteria, yeast, and viruses which all are involved in a continuous cross talk between themselves and the skin innate immune system [52–54]. Thus, healthy skin seems to be dependent on a balanced colonization, and a reduction of the diversity of the skin microbiome could be of pathophysiological relevance not only for atopic dermatitis [55], but also for other inflammatory skin conditions such as acne, rosacea, and psoriasis [56–58]. As the microbiome is now considered as our second genome, it introduces a new level of complexity that we are just starting to realize but far from being able to understand. We will have to increasingly consider the microbiome and its products in the context of an impaired epidermal barrier dysfunction since the latter allows a more facilitated interaction with our skin immune system and possibly the facilitated growth of pathogens.
5.2.1.3 Pruritus and Tissue Damage
Pruritus belongs to the main symptoms of atopic dermatitis, and it largely contributes to the impairment of the quality of life of the patients [59]. Besides this aspect, it has been shown that scratching may induce substantial tissue damage within the epidermal compartment, and this damage leads to the release of intracellular keratinocyte-derived compounds [60]. It has been assumed that these compounds are then captured by epidermal dendritic cells in the context of a microenvironment favoring a switch towards Th2, mainly provided by cytokines such as TSLP [61]. This constellation ultimately leads to the sensitization to keratinocyte-derived self-proteins to which the immune system mounts an IgE immune response [62–65]. While pruritus and scratching are not observed in the first weeks of the diseases in infancy, the first evidence for an IgE response to self-proteins is reported within the first year [66], where the chronic scratching activity has already been installed. However, it is still unclear why a number of children sensitized to self-proteins do not experience a more chronic course of the disease and the exposition to allergens like food and pollens still seems to represent the primary provocation factors in these sensitized individuals. On the other hand, a substantial proposition of adult patients with chronic and difficult to manage atopic dermatitis displays high amounts of IgE directed through self-proteins leading to the assumption that this particular population may have experienced a switch from an allergic to an auto-allergic form of atopic dermatitis (Phase 3) [1, 61].
5.2.2 Genetics of Atopic Dermatitis
The genetic background of atopic dermatitis is highly complex, but it can be schematically stratified in two main groups of genes possibly responsible for this disease (Fig. 5.1) [1]. This dichotomic view is mainly based on the fact that two main aspects learned from the pathophysiological understanding of atopic dermatitis have been put forward: an intrinsic defect in the epidermal barrier function on the one hand and a genetically driven dysfunction of the immune system leading to high tendency of IgE-mediated immune responses on the other hand.
5.2.2.1 Epidermal Genes
A number of different genes involved in the structure and regulation of the epidermal barrier functions have been proposed as candidates for the explanation of the impaired epidermal barrier function observed in atopic dermatitis. However, only two of them have reproducibly been investigated, and the functional consequences of mutations or single nucleotide polymorphisms (SNPs) of these genes have just started to be elucidated. Mutations of the gene encoding for filaggrin (FLG) have been shown to be relevant for the pathophysiology of ichthyosis vulgaris, the most common genodermatosis [67]. Similarly, genetic variants of the same gene have been shown to be associated with atopic dermatitis [68]. However, it should be noted that at least 50 different mutations or variants of filaggrin have been shown in different populations while the hot spots in European Caucasian populations seem to be different from those observed, for example, in Asian countries. Due to the relevance of filaggrin as a precursor molecule of the so-called natural moisturizing factor [69], this functional aspect is of primary interest for epidemiological studies which have supported the hypothesis that individuals carrying filaggrin mutations seem to have the highest risk to develop an early onset of atopic dermatitis followed by a severe and chronic form of the disease associated with other allergic diseases, i.e., an atopic march [38, 70–76].
Similarly, the protease-antiprotease system involved in the regulation of the epidermal barrier function has been scrutinized, and genetic variants of the gene encoding for the so-called LEKTI/SPINK5 gene has been shown to be associated with atopic dermatitis [77]. Interestingly, as is the case for filaggrin in ichthyosis vulgaris, mutations in LEKTI/SPINK5 are known to be at the origin of another genodermatosis associated with an impairment of the epidermal barrier function and the occurrence of an atopic dermatitis-like inflammation with high IgE levels: the Netherton syndrome [78]. A series of experiments performed in animal models where variants of the SPINK5 gene have been knocked in have shown that this genetic background can indeed induce an unspecific skin inflammation simply driven by an imbalance in the epidermal protease-antiprotease system [79–83]. As a consequence, this genetic background also induces a strong TSLP production by the epidermal keratinocytes and thereby prones the overall immune response towards Th2 profile. These observations could be relevant for the very initial infantile stage of atopic dermatitis (Phase 1) where an inflammatory condition is observed in the absence of any IgE sensitization context.
More recently, due to the progress in high-throughput technologies, a number of genome-wide association studies (GWAS) have been conducted in atopic dermatitis and have highlighted some new candidate genes such as C11 of 30, or ACTL9, and KIF3A [84–88]. While the functional relevance of these genes is still not understood, it is clear however that the genetic background of atopic dermatitis is even more complex than initially suspected.
5.2.2.2 Immunological Genes
Since atopic dermatitis was initially considered primarily as an immunologically driven condition, many candidate gene approaches have been performed in the past and highlighted a number of relevant genes possibly involved in the context of the immunological mechanisms underlying the IgE sensitization as well as the skin inflammation. Interestingly, several candidate genes have been identified in the so-called cytokine cluster on chromosome 5q31-33 which encompasses key mediators such as IL4, IL5, and IL13 [89–91]. Similarly, genetic variants of the genes encoding for the receptor for IL4 [92, 93] have reproducibly been described as well as mutations in the promoter region for the gene and encoding for the chemokine CTACK/CCL27 and particularly the keratinocyte-derived cytokine TSLP which seems to play a key role in the pathophysiology of the disease [94–97]. The immunological aspects underlying atopic dermatitis have been reviewed recently [98] highlighting the move away from a uniform dogma involving mainly the Th1–Th2 balance towards a more complex network of many different T cells with a wide spectrum of cytokines such as IL-22 [99–102], IL-9 [103–105], and IL25 [106–109].
When it will come to establish a genomic profile supposed to be more or less characteristic for a subgroup of patients with atopic dermatitis, a various combination of different candidate genes will certainly be considered and will hopefully give us more information about the individual risk for the disease itself and the associated atopic march.
5.2.3 Epigenetics in Atopic Dermatitis
In the recent years, it became evident that the classical genetic background can be substantially modulated by mechanisms involving the methylation of genes and the acetylation/deacetylation of chromatin regions or microRNA sequences. Thus, since these mechanisms are under the significant influence of environmental factors such as foodstuff, exposition to pollutants, or potentially signals from the microbiome, epigenetic regulation could explain some contradictory results of a number of genetic studies reported in the field of allergic diseases. Only few studies have addressed the epigenetic regulation in atopic dermatitis [110–114], but it is expected that new knowledge in this field could provide important information with regard to new prevention strategies relevant for individualized prevention measures.
5.3 Heterogeneity of the Clinical Phenotype of Atopic Dermatitis
The clinical phenotype of atopic dermatitis displays a very wide spectrum of different aspects, and at least four levels of complexity can be distinguished, namely, (1) the age of onset, (2) the natural history, (3) the semiology of the lesions, and (4) the severity of the disease. These aspects are not independent but are usually related and sometimes strongly interacting.
5.3.1 Age of Onset of Atopic Dermatitis
As shown on Fig. 5.2, at least six different ages of onset can be identified [115]. It is currently far to be clear what the reasons for this high heterogeneity in the age of onset could be and whether genetic or more environmental factors could play a major role in the initiation of the study. Overall, it is interesting to note that the sensitization rate is the highest among people having started the disease before adulthood, while the classical late onset (between 20 and 60 years) seems to be a particular form which affects mainly females in which an IgE sensitization as well as other atopic diseases such as rhinitis or asthma are rarely seen [116–122]. Interestingly however, as it seems the case for psoriasis, we are currently assisting to the emergence of a very late onset (senile) of atopic dermatitis which is characterized by an average age of onset of more than 65 years, a rather severe form of atopic dermatitis typically as an erythrodermic variant, a male predominance (ratio M:F of 3:1), and a mean high total IgE level above 8,000 kU/l [123–126].
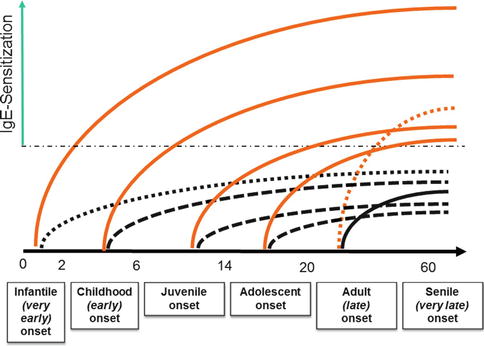

Fig. 5.2
The different types of onsets observed in atopic dermatitis (Adapted from [115]) and the risk of IgE sensitization
5.3.2 The Natural History of Atopic Dermatitis
Figure 5.1 depicts the classical natural history of a child with an infantile or childhood onset of atopic dermatitis in which the sensitization emerges during the chronic phase of the disease and potentially ending up with other atopic diseases affecting the airways, i.e., allergic rhinitis and allergic asthma. This natural history affects approximately 45 % of all patients, and they usually suffer from the more severe forms of the disease. On the other hand, it has been reported that approximately 60 % of the kids will improve the condition and go into complete remission before the puberty [18, 19, 115, 127–132]. However, this has been questioned more recently in a large cross-sectional and cohort study which suggests that mild to moderate forms of atopic dermatitis may persist well into the second decade and even longer, strongly suggesting that atopic dermatitis should be considered as a lifelong condition [133].
5.3.3 Semiology of the Skin Lesions
Although atopic dermatitis is usually characterized by more or less sharply demarcated, erythematous, and scaly lesions mainly localized on typical body sites such as the head, neck, flexures, and hands, this archetypical kind of morphology may not be applied to all patients suffering from this disease (Fig. 5.3) [117]. Indeed, besides clear evidences of dry skin (xerosis), the semiology of the skin lesions can also be very diverse including, e.g., isolated retroauricular fissures or mainly highly infiltrated and lichenified lesions on other places, sometimes isolated or combined with a strong pruritus (the so-called lichen Vidal). Finally, the so-called nummular eczema is also considered as a possible variant of atopic dermatitis for which, besides the possible role of the microbiome and especially Staphylococcus aureus [134], no real pathophysiological explanation has been found so far.
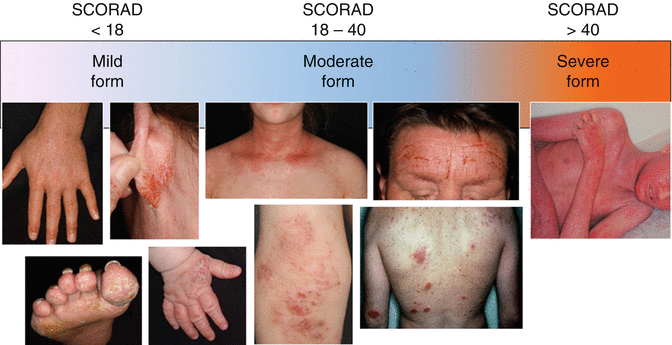

Fig. 5.3
The heterogeneity of the clinical phenotype of atopic dermatitis in relation to the severity grade
5.3.4 Spectrum of the Severity in Atopic Dermatitis
One of the key features of atopic dermatitis remains its wide spectrum of severity, which can be evaluated by different tools. Among the validated tools currently in use in clinical practice as well as in the context of clinical trials are the scoring systems SCORAD [135–139] and EASI [140–142]. Moreover, due to the increased importance of patient-related outcomes under socioeconomic and pharmaco-economic aspects, tools to evaluate the severity from the patients point of view (PO-SCORAD and POEMS) [136, 141, 143–147] as well as questionnaires for the evaluation of the quality of life specifically designed for patients with skin conditions (e.g., DLQI or the Skindex) have been developed [148–156].
Three main severity categories have been defined: mild (including xerosis), moderate, and severe. It has been shown that most of the patients are switching from one category to the other depending on the disease activity, usually dictated by the flares. As expected, disease severity is strongly associated to the frequency of flares. However, in the absence of adequate therapeutic management, the disease keeps a level of severity of its own. Except for the case of the infantile and childhood onsets of the disease where a gradual increase in the affected body regions and body surface area is usually observed during the chronic course of the disease, only few data are available for the natural history in terms of severity with the adult onsets of atopic dermatitis. Among the population of patients suffering from atopic dermatitis, the severity is not evenly distributed, but it is estimated that severe patients represent 10–15 % of the overall AD populations. However, the moderate-to-severe and severe population (SCORAD >30) have the biggest medical unmet need.
5.4 The Quest for Biomarkers Leading to a New Taxonomy of Atopic Dermatitis
The spectrum of possible biomarkers useful in any kind of disease is quite large and much dependent on the kind of disease and the approach selected. Progress in our understanding of the genetic background and the epidemiology and pathophysiology of atopic dermatitis has led to a series of candidate genes and molecules which could be used as biomarkers in the context of personalized medicine. With regard to the particular natural history of atopic dermatitis, a number of different kinds of biomarkers are expected to be discovered. Figure 5.4 shows the typical scenarios of a natural history of atopic dermatitis with early onset and the different time points at which biomarkers could be useful for the management of the disease. Thereby, the following types of biomarkers can be considered:
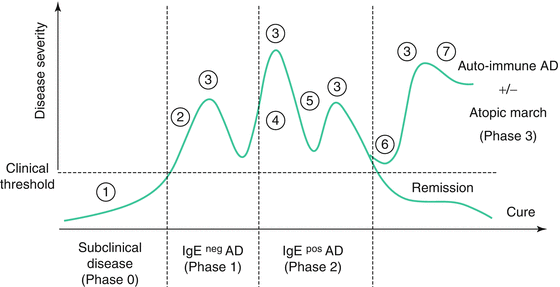

Fig. 5.4
Schematic profile of the natural history of atopic dermatitis with the different time points where a biomarker could be applied (Adapted from [178]). 1 Screening biomarkers for detection of patients at risk for atopic dermatitis. 2 Diagnostic biomarkers. 3 Severity biomarkers. 4 Biomarkers for individualized diagnostics of the sensitization profile. 5 Pharmacogenomic biomarkers predicting the therapeutic response. 6 Prognostic biomarkers predicting the course (complete remission or relapse) of the disease. 7 Diagnostic biomarkers for autoimmunity in atopic dermatitis
1.
Screening biomarkers which are unable to identify those patients at high risk of developing atopic dermatitis even before the first clinical signs of the disease.
2.
Diagnostic biomarkers which could be used at a very early time point in case of differential diagnostic problems.
3.
Severity biomarkers typically useful in the setting of clinical trials for the evaluation of therapeutic success or even as surrogate biomarkers for clinical trials and long-lasting control.
4.
Biomarkers for individualized diagnostics of the sensitization profile. A determination of specific IgE belongs to this kind of biomarkers already available.
5.
Biomarkers predicting the therapeutic response and the risk of side effects for a specific drug (pharmacogenomics).
6.
Prognostic biomarkers which may predict the occurrence of remission before puberty or adulthood or a successful disease-modifying strategy.
7.
Diagnostic biomarkers for atopic march and/or autoimmunity in atopic dermatitis enabling to identify those patients who would not have benefit from any kind of avoidance strategy with respect to allergens or other environmental factors.
Overall, it is assumed that most of the diseases will not be stratified according to one single biomarker [157–159]. Thus, as our understanding in the genetics and pathophysiology of atopic dermatitis will progress, an increasing number of different biomarkers will be available, and depending on the goal of the stratification, a more or less complex combination of several biomarkers will be considered: they built the so-called endophenotypes. As for other complex diseases, the endophenotype will ultimately lead to a stratification of atopic dermatitis according to a new kind of molecular taxonomy [160]. This stratification will be the basis of future developments in personalized prevention and therapy.
5.5 The Long Way to Personalized Management of Atopic Dermatitis
With regard to the abovementioned complexity of the pathophysiology and clinical phenotype of atopic dermatitis, a number of opportunities can be defined for which a personalized approach could substantially be of benefit for an endophenotype-defined subgroup of patients suffering from this disease.
In the following, a few ideas and speculations are drawn in order to address some key opportunities and goals.
5.5.1 Personalized Prevention to Avoid the Atopic March
The analysis of different kinds of natural history of this disease has strongly suggested that there is one particular subpopulation of patients starting with an early onset which has the highest risk to develop a long life severe form of atopic dermatitis associated with strong sensitization and a high risk to develop allergic asthma, i.e., the atopic march. Preliminary evidence from genetic studies suggests that filaggrin mutations are strongly associated with this particular natural history of the disease [70–73, 161]. However, we will certainly need some other biomarkers related to the immunological sets of genes associated with the regulation of IgE synthesis in order to have a more complete picture of the possible endophenotype associated with individuals at high risk to develop this particular course of the disease. Based on this information provided by a combination of appropriate biomarkers, it could be possible to identify the newborns and children at high risk at a very early time point (Phase 0) and to stratify these populations starting in a way that would allow to provide a more differentiated information to the parents as well as the very early use of particularly adapted prevention measures [162]. This kind of primary prevention could potentially hamper the appearance of the disease or lead to the delay of the first symptoms. Appropriate prevention measures with regard to the exposition to foodstuff and other potential allergens could be designed in order to induce tolerance instead of sensitization. Even if these primary prevention measures would not be effective, and the disease has already started (Phase 1), the knowledge about the risk of atopic march would be very helpful in order to convince the parents about the importance of a good compliance to a therapeutic management plan proposed by the physicians. In this context, a targeted implementation of a long-term proactive management would be of particular benefit and could potentially hamper the emergence of sensitization, i.e., the Phase 2 of the disease.
5.5.2 A Personalized Management to Control a Disease on the Long Run
While the prevention of the atopic march is a strategy which is applicable in infancy and childhood, it would be important to design new approaches for a better control of the flares in patients suffering from a chronic and moderate-to-severe form of atopic dermatitis. Indeed, although it is now well accepted that the control of the flares is mainly reached by a better control of the subclinical information, it is still not clear how long the proactive management has to be provided in order to reach the goal of a complete remission of the disease. Thus, appropriate biomarkers would give us important information about the subclinical inflammation and the time point which should be reached in order to stop the long-term anti-inflammatory treatment.
5.5.3 A Personalized Diagnostic Approach to Identify the Provocation Factors in Atopic Dermatitis
Most of the patients affected by atopic dermatitis display a very wide spectrum of sensitization as detected by either prick test or specific IgE. However, it is well accepted that only a few of these allergens may be relevant for this particular patient [163]. This issue is not specific to atopic dermatitis but remains a challenge for most allergic diseases when allergen-specific immunotherapy is envisaged. Therefore, a refinement of the allergic diagnostic based on new molecular approaches could be very useful in order to detect those allergens which are relevant for each individual patient in order to provide him an appropriate avoidance strategy and thereby to reduce the number of provocations and flares due to contacts with allergens. Most importantly, the hotly debated causative therapy with allergen-specific immunotherapy for atopic dermatitis would have a greater chance of success under these conditions [164–171].
5.5.4 Personalized Approach in the Context of Drug Development
Patients affected by moderate-to-severe atopic dermatitis usually cannot be controlled efficiently on long term using the few approved systemic drugs for these conditions such as cyclosporine. Therefore, a number of patients have to be treated with off-label regimens [172–176]. However, all these treatments which have not been developed specifically for this disease may expose the patients to unwanted, more or less severe side effects. Therefore, the risk-to-benefit ratio for most of the patients treated by systemic immunosuppressive drugs is not satisfactory. A better understanding of the complex pathophysiology of atopic dermatitis and more specifically the role of individual cytokines in the regulation of IgE and in the generation of the skin inflammation in patients with the more severe forms will have a tremendous impact on the discovery of new biomarkers and potentially on the development of new targets for this particular population. Among the biologics which are currently in development, the anti-IL4 strategy seems to be the most promising [177], while it is not clear whether this particular approach will be beneficial only for a subpopulation of patients with AD.
5.6 Conclusion
Due to its pathophysiological and clinical complexity, atopic dermatitis is a candidate disease for personalized medicine. Although atopic dermatitis is not life-threatening, it is well accepted that it kills the quality of life of the patients and their relatives. Unfortunately, more than other kinds of disease, atopic dermatitis has so far only been treated with some few standard regimens including emollients and topical anti-inflammatory drugs such as steroids and calcineurin inhibitors. For the most severe forms, depending on the countries, there is no approved systemic drug for the management of this disease. On the other hand, the heterogeneity of the clinical phenotype and our knowledge about the natural history strongly suggest that the management of these patients should be considered in a more differentiated way. While about half of the children affected by atopic dermatitis will experience a remission until adulthood, the other half will have to suffer more or less from this disease for their whole life. Moreover, within this population, a substantial proportion of patients will develop other atopic diseases like rhinitis and allergic asthma, and there is an urgent need and opportunity for early intervention strategies in order to limit the emergence of the atopic march. Thus, one of the primary goals of personalized medicine in the context of atopic dermatitis is to develop new disease-modifying strategies aimed to better control the inflammation in the skin and ideally to hamper the occurrence of IgE sensitization in those patients with high risk to develop an atopic career.
References
2.
Flohr C, Mann J. New insights into the epidemiology of childhood atopic dermatitis. Allergy. 2014;69(1):3–16. Epub 2014/01/15.PubMed
3.
Beikert FC, Langenbruch AK, Radtke MA, Kornek T, Purwins S, Augustin M. Willingness to pay and quality of life in patients with atopic dermatitis. Arch Dermatol Res. 2014;306(3):279–86. Epub 2013/08/29.PubMed
4.
Beattie PE, Lewis-Jones MS. An audit of the impact of a consultation with a paediatric dermatology team on quality of life in infants with atopic eczema and their families: further validation of the Infants’ Dermatitis Quality of Life Index and Dermatitis Family Impact score. Br J Dermatol. 2006;155(6):1249–55.PubMed
5.
Zuberbier T, Orlow SJ, Paller AS, Taieb A, Allen R, Hernanz-Hermosa JM, et al. Patient perspectives on the management of atopic dermatitis. J Allergy Clin Immunol. 2006;118(1):226–32.PubMed
6.
Healy E, Bentley A, Fidler C, Chambers C. Cost-effectiveness of tacrolimus ointment in adults and children with moderate and severe atopic dermatitis: twice-weekly maintenance treatment vs. standard twice-daily reactive treatment of exacerbations from a third party payer (U.K. National Health Service) perspective. Br J Dermatol. 2011;164(2):387–95. Epub 2010/11/19.PubMed
7.
Mancini AJ, Kaulback K, Chamlin SL. The socioeconomic impact of atopic dermatitis in the United States: a systematic review. Pediatr Dermatol. 2008;25(1):1–6.PubMed
8.
Jenner N, Campbell J, Marks R. Morbidity and cost of atopic eczema in Australia. Australas J Dermatol. 2004;45(1):16–22.PubMed
9.
Lamb SR, Rademaker M. Pharmacoeconomics of drug therapy for atopic dermatitis. Expert Opin Pharmacother. 2002;3(3):249–55.PubMed
10.
Herd RM. The financial impact on families of children with atopic dermatitis. Arch Dermatol. 2002;138(6):819–20.PubMed









