Fig. 42.1
Anogenital warts (condyloma acuminata)
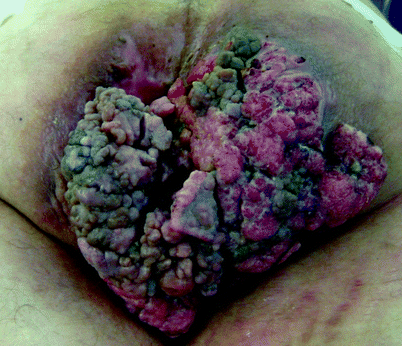
Fig. 42.2
Giant condyloma acuminata (Buschke-Lowenstein tumor)
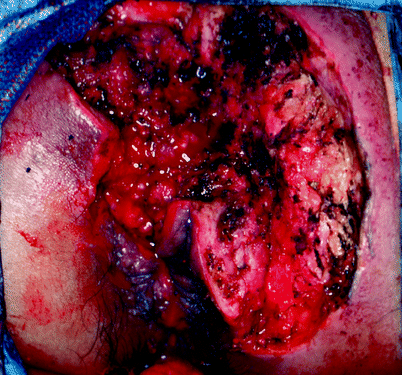
Fig. 42.3
Tissue defect after wide excision of giant condyloma acuminata (Buschke-Lowenstein tumor)
Split-thickness skin grafts may be inadequate to manage the perineal wounds that result during the course of the management of perineal hidradenitis suppurativa (Fig. 42.4) or Fournier’s gangrene (Fig. 42.5). Treatment of these two conditions often results in perineal wounds characterized by large tissue defects and local tissue induration and infection. After debridement of all necrotic and infected tissue, it may be determined that healing by secondary intention or coverage with split-thickness grafts is inadequate (Fig. 42.6). In such cases, flap coverage may be necessary [5, 6].
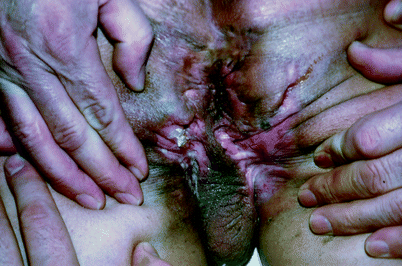
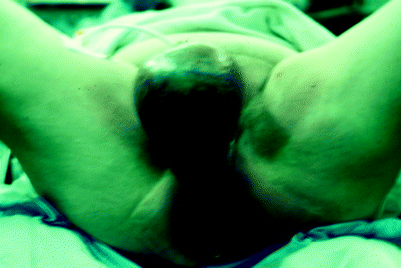
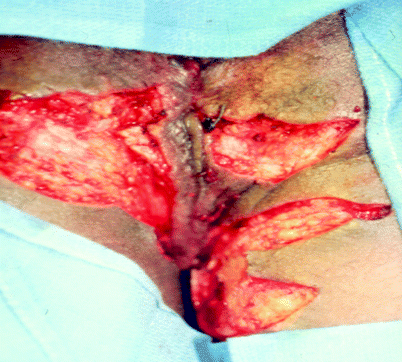

Fig. 42.4
Hidradenitis suppurativa

Fig. 42.5
Fournier’s gangrene

Fig. 42.6
Tissue defects after excision and debridement ofhidrandenitis suppurativa
When flap coverage is necessary for the reconstruction of these complex perineal wounds, successful reconstruction requires the recruitment of locoregional, well-vascularized tissue [7–9] that can take a simple random pattern construction dependent upon the local circumstances (Fig. 42.7a–h). A multitude of more complex flap options can, however, be employed depending on the specific wound characteristics and reconstructive requirements. The majority of cases encountered by the colorectal surgeon are those resulting from extirpation of low rectal or anal carcinomas (Figs. 42.8 and 42.9). Complex reconstruction techniques may be especially necessary in patients who require, or have previously been subjected to, adjuvant radiation therapy. Most of these defects are appropriately managed with a vertical rectus abdominis myocutaneous (VRAM) flap (Fig. 42.10) or either unilateral or bilateral gracilis myocutaneous flaps. These locoregional flaps can be reliably rotated from their normal anatomic location to reconstruct complex perineal defects while maintaining circulation via their dominant blood supply. The cutaneous portion of the myocutaneous flap can be utilized to resurface substantial soft-tissue deficiencies. The flap itself provides bulky tissue to obliterate dead spaces. These relatively remote tissues are naturally located outside of perineal radiation portals, and therefore rotation of these flaps into previously irradiated wounds delivers vascularized tissue to relatively ischemic wound beds, facilitating healing [10, 11]. In addition, myocutaneous flaps may be relatively resistant to the bacterial contamination commonly found in perineal wounds [10–13].
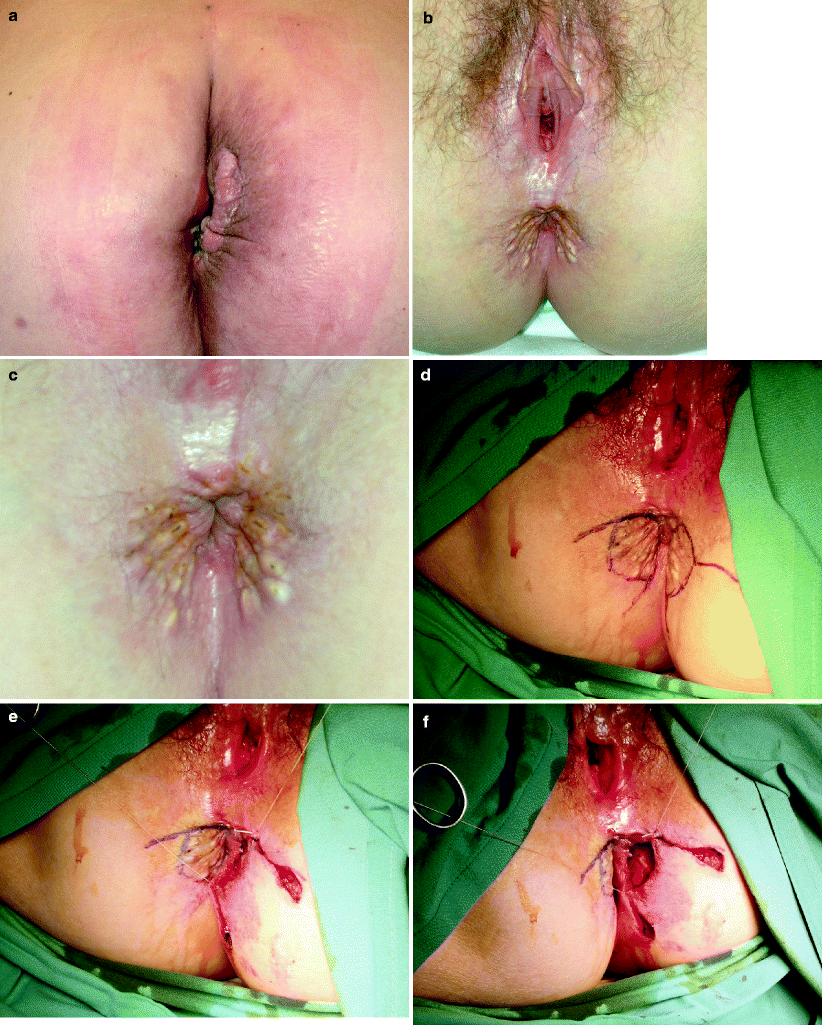
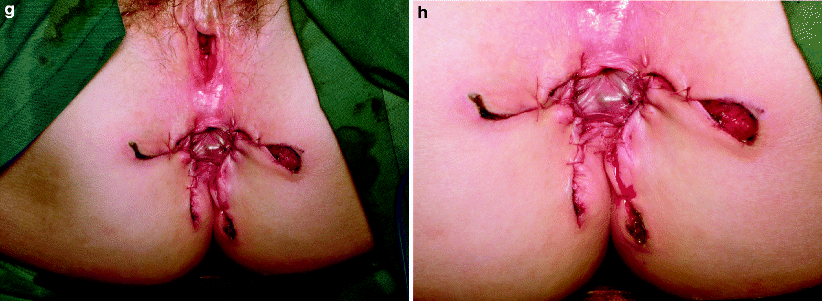
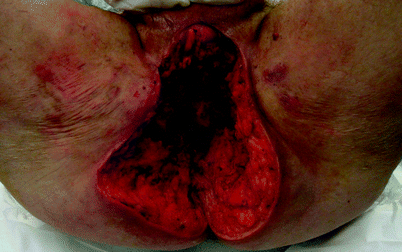
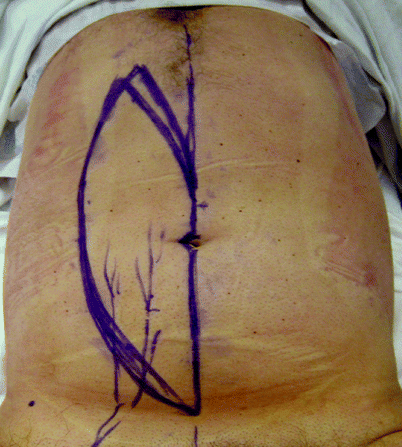
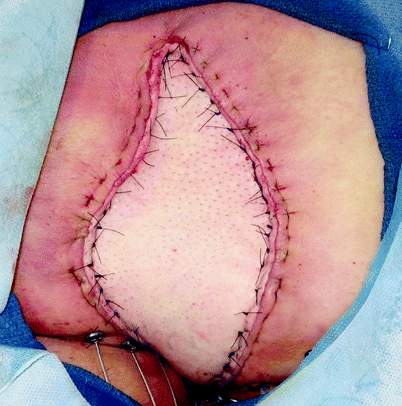


Fig. 42.7
(a) Recurrent carcinoma in the scar of a perineal wound after abdominoperineal resection for rectal cancer. (b–h) Perianal Paget’s disease covered by a customized advancement flap after excision (Courtesy of A. P. Zbar)

Fig. 42.8
Tissue defect after extirpation of the recurrent carcinoma

Fig. 42.9
Planned vertical rectus abdominis myocutaneous flap

Fig. 42.10
Tissue defect closed with vertical rectus abdominis myocutaneous flap
General plastic surgical reconstructive principles include restoration of form and function while minimizing donor-site morbidity. Overall patient safety is also of paramount importance. Both gracilis and rectus abdominis myocutaneous flaps can be harvested with relatively low donor-site morbidity. Flap characteristics vary depending on individual patient body habitus. Surgical techniques have been well described, and applications in perineal reconstruction are well founded [14–18].
The inferiorly based VRAM flap is maintained on the deep inferior epigastric artery and vein. The rectus abdominis muscle originates from the pubis and inserts on the costal margin. Perforating vessels course from the main vascular pedicle, on the undersurface of the muscle and through the muscle, supplying the overlying skin and subcutaneous fat [19




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








