Fig. 37.1
Chest X-ray demonstrating a large mediastinal haematoma as typically seen in a penetrating injury of the large mediastinal vessels
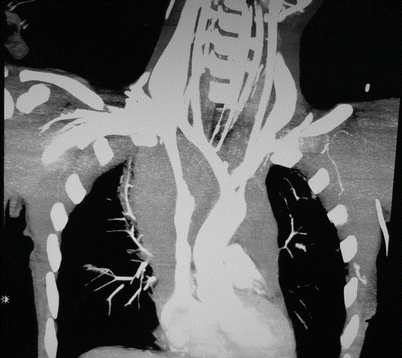
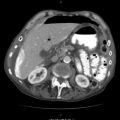
Fig. 37.2
CT angiogram confirming a large mediastinal haematoma
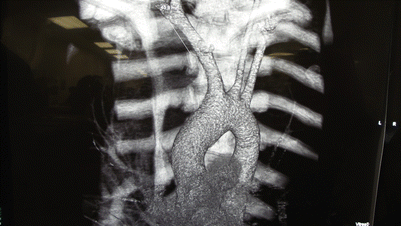
Fig. 37.3
CT angiogram (with reconstruction) demonstrating a penetrating injury to the innominate artery
A few practical guidelines in dealing with these patients follow:
Always ensure universal protective gear when treating these injuries as a stable situation could at any time change into a spurting arterial bleed once successful fluid resuscitation becomes effective.
If at all possible, install cell-saving measurements with autotransfusion in view of the massive possible blood loss encountered, thereby preventing massive bank blood transfusions.
Keep these patients warm with adequate fluid resuscitation and constant effective communication with the anaesthetic team in order to avoid the deadly triad of hypothermia, lactic acidosis and coagulopathy.
Many of these injuries need to be managed via a posterolateral thoracotomy with the patient in lateral decubitus position, bearing in mind that a hypovolemic patient can rapidly decompensate when changed from the supine to decubitus position requiring adequate and ongoing fluid resuscitation prior to and during turning as well as rapid surgical entry into the thorax. It is often beneficial to have the theatre sister prepared with all surgical instruments and scrubbed prior to turning the patient.
The smaller undiagnosed and undrained pneumothoraces of penetrating injuries can be converted into tension pneumothoraces by adhesive sterile surgical drapes. These may require urgent chest tube drainage or needle decompression if clinical monitoring shows haemodynamics to worsen soon after these drapes were applied. Have a low index of suspicion for these sudden changes, and monitor the patients constantly and very carefully.
Always have internal defibrillator pads available for internal cardiac defibrillation in these unstable patients. Whenever previous sternotomy or thoracotomy scars are present in trauma patients, add external adhesive defibrillator pads prior to sterile draping as entry into the thorax can be more cumbersome due to adhesions of previous surgery, and access to the heart for internal defibrillator pad usage may therefore be limited or delayed.
Once the mediastinal vessel injury is definitively managed, always consider injuries to adjacent mediastinal structures like the trachea, bronchia or oesophagus.
Endovascular management of some of these large vessel injuries (e.g. the descending thoracic aorta) is an evolving science in unstable trauma patients but should definitely be considered should such expertise be available in the treating hospital.
37.3 Incisions and Exposure
These injuries are mainly managed via median sternotomy, anterolateral thoracotomy or posterolateral thoracotomy. The details of these techniques are described in “Penetrating Cardiac Trauma (Anterolateral Thoracotomy)” and “Pulmonary and Bronchotracheal Trauma (Posterolateral Thoracotomy)” chapters.
37.4 Management of Specific Injuries
37.4.1 Injuries to the Ascending Aorta
Bleeding from penetrating trauma to the ascending aorta results in pericardial tamponade due to the fact that it is anatomically situated inside the pericardial sac. Emergency median sternotomy should be performed and the sternal retractor opened. A tense pericardium due to tamponade can be difficult to grab for pericardiotomy. Use a scalpel blade to make a small incision, and then quickly open the pericardium vertically in the midline with scissors, and create lateral perpendicular extensions at the inferior, diaphragmatic part of the pericardial incision. This should give the pericardiotomy the shape of an inverted “T” which usually provides best exposure. Manually evacuate the pericardial content causing the tamponade, and assess cardiac contraction and rhythm status. If asystole or ventricular fibrillation is present, internal cardiac massage should be maintained until a perfusing rhythm and adequate cardiac filling volume can be established. Communicate closely with the anaesthetic team with appropriate administration of resuscitation drugs like Adrenalin throughout. Internal defibrillation paddles can be used to defibrillate a non-perfusing ventricular rhythm but should be avoided in asystole. Find the bleeding laceration on the ascending aorta, and control it with digital compression or a side-biting clamp (Satinsky or large Wiley “J” clamp). Once temporary haemostasis is achieved in this manner, use limited (but well-spent) time with attention to exposure and planning the repair. Pericardial retraction sutures improve access to the ascending aorta. Negotiate mentally whether repair underneath a digitally compressing finger or a carefully applied side-biting clamp will be most effectively performed. If a side-biting clamp is used, ensure adequate residual patency of the underlying aorta for proper ejection of the heart to perfuse the head and neck vessels. Consider the type of injury or defect and appropriate repairing strategy using 3.0 or 4.0 monofilament nonabsorbable sutures. If a small pinpoint penetrating hole is encountered, a pledgeted “U” suture (autologous pericardial or Teflon felt pledget) is most effective for repair. In a larger defect (but where the edges can be approximated), a lateral aortorrhaphy will suffice using direct suturing with a double layer of first a horizontal mattress suture technique, ensuring that the endothelium is caught with every suture in order to traverse the full-thickness aortic wall and especially the strong adventitial layer with the suturing needle. This technique should evert full thickness of 2–3 mm of the aortic wall in the first layer, which is then followed with a second layer of over-and-over suturing while ensuring that the bites of the second layer are just fractionally more superficial than the first layer in order to avoid creating new suture holes. A larger defect where edges cannot be approximated should be repaired with the use of an autologous pericardial patch or prosthetic material (e.g. Dacron or Gore-Tex). The pericardium can easily be harvested from the pericardiotomy site and should be used untreated (kept moist in a saline-soaked Ratex swab after harvesting) with the smooth aspect of the pericardium applied to the internal aspect of the aorta. While sutures are tied on the aorta, it is ideal that the systolic blood pressure should be carefully lowered. A skilled anaesthetist may be able to perform this formidable task in an unstable trauma patient, but the easiest way is usually to manipulate the head-up bed position for a few seconds prior to tying a suture in order to achieve temporary but very reversible mild hypotension. Complete cross clamping of the ascending aorta should be avoided if at all possible in view of the disastrous complication of cardiac distension with irreversible distension injury to the left ventricle. Should aortic cross clamping be necessary, it should be accompanied by inflow occlusion of the superior vena cava (SVC) and inferior vena cava (IVC) prior to the aortic cross clamp in order to empty the heart and prevent over-distension injury. These are formidable manoeuvres that could have very serious side effects and should not be performed without careful consideration and should only be performed in inexperienced hands. It serves no purpose to have a repaired ascending aorta but an irreversibly injured myocardium.
Whenever an anterior injury to the ascending aorta was encountered, the posterior surface should always be checked in view of a possible through-and-through injury. A posterior injury is far more challenging to repair but could be managed with careful mobilization of the distal ascending aorta. Carefully divide the tissue between the aorta and pulmonary artery with a diathermy on low settings (staying on the aortic aspect of this tissue and avoiding injury to the thin-walled pulmonary artery). Pass a Curly-Semb vascular clamp behind the aorta in the transverse sinus, and pull a wet umbilical tape through the transverse sinus looped around the distal aorta (Figs. 37.4 and 37.5). Give this to the assistant to use as a handle to pull the aorta away from the superior vena cava. The same could be performed more proximally on the aorta, and in this manner, reasonable retraction with exposure of the posterior surface can be achieved. Be careful to narrow the aortic lumen too aggressively as cardiac output will be compromised, and constant communication with the anaesthetic team is imperative. The same principles for repair apply. In cases where adequate exposure of the posterior aortic surface cannot be achieved, cardiopulmonary bypass will be required in order to maintain circulating volume and create a bloodless field. The posterior surface of the aorta can be packed well with Ratex swabs and tamponade bleeding in this manner until cardiopulmonary bypass equipment can be organized.
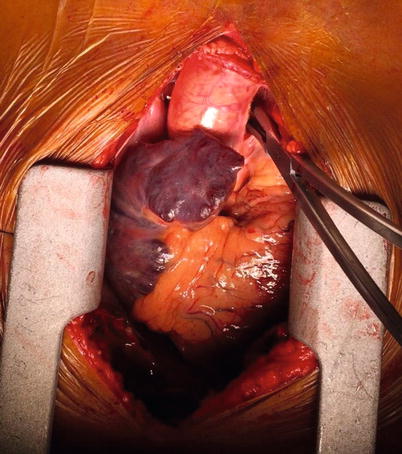
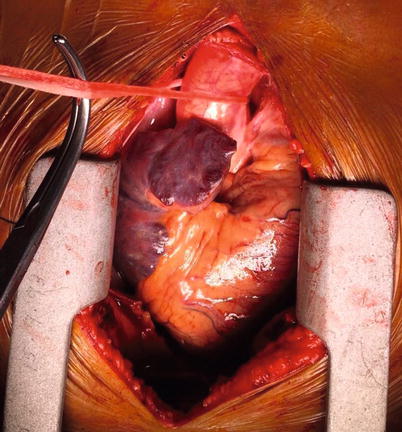

Fig. 37.4
Curly-Semb vascular clamp passed behind the ascending aorta in the transverse sinus

Fig. 37.5
Umbilical tape looped around the ascending aorta in order to aid retraction
37.4.2 Injuries to the Aortic Arch
Stable patients with contained injuries of the aortic arch are far safer repaired using cardiopulmonary bypass with the introduction of deep hypothermic cardiac arrest, a bloodless field and physiological cerebral protection. Unfortunately in the acute trauma setting, these adjuncts are rarely available. The same principles apply in the methods of repair as for the ascending aorta, but most importantly, the chances of a successful repair are greatly enhanced by optimal exposure. Extend the median sternotomy cervically. The thymus tissue can be wiped aside from the midline with an abdominal swab, but the venous drainage of the thymus needs to be carefully ligated from the innominate vein with preferably a Ligaclip. These unimpressive thymic veins are small and shut down in hypovolemic trauma patients but open up with reperfusion and adequate resuscitation and have been the reason for many relook sternotomies due to bleeding after an initial successful operation. The innominate vein can be looped with a vascular tape and pulled in a cephalic direction in order to expose the aortic arch better (Fig. 37.6), or if exposure is still inadequate, it can be double ligated, divided and retracted. Most importantly, the aortic arch is anatomically situated outside the pericardium, and in the presence of an anterior mediastinal haematoma, tissue dissection can lead to a false dissection plane within the aortic wall (between adventitia and media) resulting in an iatrogenic dissection injury. Approach to the aortic arch should be initiated from within the pericardial sac with accurate differentiation between haematoma and adventitia. Be prepared to manage inflow occlusion of the superior and inferior vena cava and ascending aorta with a short-term cross clamping. It is far safer to loop the ascending aorta and both cavae in anticipation of inflow occlusion than to be managing it amidst exsanguinating circumstances. Looping of the ascending aorta with an umbilical tape can be performed as described above. For looping of the superior vena cava (SVC), the soft tissue medial to the SVC (between the SVC and the aorta) must be lifted with a forceps and incised with scissors. An O’Shaughnessy or Lahey forceps is passed behind the SVC from lateral to medial (taking careful consideration of the fact that the right pulmonary artery branch is just deep to that and the azygos vein enters the SVC from posterior) and used to pull a wet umbilical tape through to loop it. (Fig. 37.7). For looping of the inferior vena cava (IVC), a long pair of scissors is used to develop the area between the right inferior pulmonary vein and the IVC. A Curly-Semb vascular clamp is passed from medial to lateral and used to circumnavigate the IVC by pulling a loop of umbilical tape through. Principles of repair are the same for the ascending aorta.
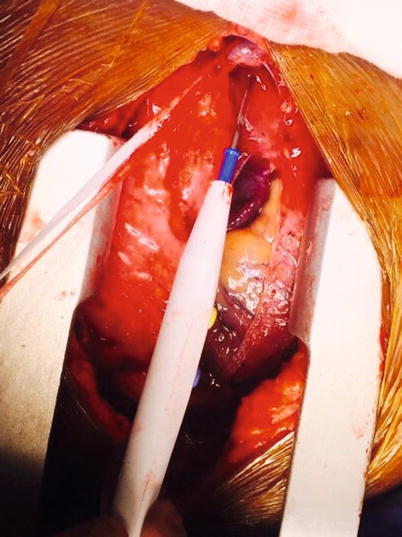
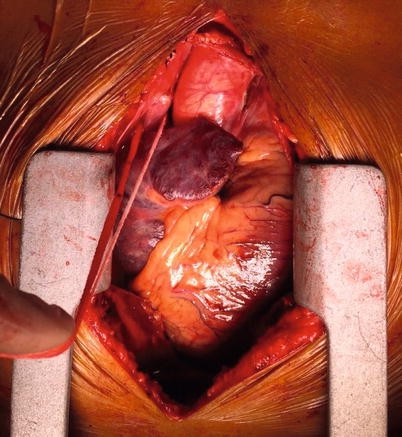

Fig. 37.6
A looped innominate (or brachiocephalic) vein being pointed at by a surgical diathermy

Fig. 37.7
A looped SVC (with wet umbilical tape) prepared to be clamper for inflow occlusion
37.4.3 Injuries to the Branches of the Aortic Arch
It is imperative that the surgeon treating injuries to the branches of the aortic arch know and understand the possible anatomic anomalies to be encountered. A bovine arch (where both the innominate and left common carotid arteries have a common origin from the aortic arch) can be encountered in 5–30 % of cases depending on the treated population. This may complicate repair, but a clear anatomic understanding should result in accurate correction of the injury.
Approach to the branches of the aortic arch is enabled via longitudinal cervical extension of the median sternotomy or supraclavicular extension to the right for the innominate artery injury or to the left for left common carotid injury. Division of the strap muscles of the neck from its insertion point into the sternum gives improved exposure of the carotid sheath. Carefully dissect the arch vessels free, and avoid the area of injury until proximal and distal control is achieved. The proximal right subclavian artery (SCA) should be approached with extra care as the vagus nerve with recurrent laryngeal branch hooks around the proximal 1.5–3 cm of the right SCA after the bifurcation of the innominate artery. Digital pressure control of active bleeding is essential until proximal and distal control is achieved. Contained injuries at the base of the innominate or left common carotid arteries (where it originates from the arch) are managed by far superiorly with cardiopulmonary bypass and deep hypothermic cardiac arrest or contralateral axillary artery cannulation for cerebral perfusion above a primary end-to-end anastomosis. If these adjuncts are not readily available and a life-threatening bleeding is at hand, primary repair should be performed with Satinsky or Wiley “J” vascular clamps of the arch and distal control. No temporary shunts are needed in these vessels as adequate cerebral crossover flow usually exists in younger trauma patients and can be more time-consuming to achieve in the acute situation and unnecessary in the controlled situation where cardiopulmonary bypass could be obtained.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree