Ambulatory surgery is commonplace for a multitude of procedures and a wide range of patients. The types of procedures performed in the ambulatory setting are becoming more work-intensive, and patients with comorbidities make for a challenging environment. For a safe environment for surgery in ambulatory facilities, the complex task of patient selection is necessary. Until an algorithm is created that includes provider, procedure, facility, and patient comorbidites, clinicians must rely on general guidelines rather than precise recommendations.
Key points
- •
Ambulatory surgery is commonplace for a multitude of procedures and a wide range of patients.
- •
The types of procedures performed in the ambulatory setting are becoming more work-intensive, and patients with comorbidities make for a challenging environment.
- •
For a safe environment for surgery in ambulatory facilities, the complex task of patient selection is necessary.
- •
Until an algorithm is created that includes provider, procedure, facility, and patient comorbidities, clinicians rely on general guidlines rather than precise recommendations.
- •
When determining if a patient is suitable for ambulatory surgery, multiple factors must be assessed.
Ambulatory surgical volume in the United States increased 300% between 1992 and 2006. In 2006, an estimated 53.3 million procedures were performed in ambulatory centers, 19.9 million in hospitals, and 14.9 million in freestanding surgical centers. The increase has occurred partly because of financial incentives, changes in clinical practice from advances in technology, and patient expectations. Whether a case is appropriate for the ambulatory setting depends on where the surgery will occur, the personnel involved, the surgical procedure, and the patient’s medical status. Consideration of these 4 criteria will help achieve optimal patient outcomes.
Ambulatory surgery can occur in a physician’s office, freestanding ambulatory surgical center, building on a medical campus, or hospital. In the hospital setting, ambulatory procedures can be consolidated in one location or interspersed with inpatient procedures. Transportation between the procedural facility and a hospital for additional postoperative care also must be considered, and a transfer process should be in place. A facility that is accredited by the American Association for Accreditation of Ambulatory Surgery Facilities, the Accreditation Association for Ambulatory Health Care, or the Joint Commission is essentially obliged to operate in a manner consistent with the American Society of Anesthesiologists (ASA) standards and guidelines. In addition to the physical plant, the available equipment and supplies will also influence what procedures may be performed. The ASA and others have set expectations for ambulatory anesthesia in the form of standards and guidelines that apply independent of location. For example, the ASA and the Agency for Healthcare Research Quality recommend that equipment for standard monitoring include a noninvasive blood pressure monitor, a means to record heart rate and respiration, an electrocardiograph, and a pulse oximeter. The ASA adds continuous monitoring of end expiratory carbon dioxide and the ability to measure temperature, if indicated.
Safety is a continuum from the preoperative to the postoperative phase of care; at the time of discharge, patients should received written postoperative instructions and be discharged to the care of a responsible adult. Ideally, when surgery is performed in a freestanding ambulatory surgery center or office, the surgeon performing the surgery should have credentials to perform that procedure in a hospital and should be operating within the scope of his specialty training.
Another consideration is the personnel staffing the center. Will anesthesia be required, and if so, how much and administered by whom? Will a registered nurse be sufficient if anesthesia is given as a local injection with moderate sedation, or will the services of an anesthesiologist or a certified registered nurse anesthetist (CRNA) be needed? One study showed that anesthesia provided by nonanesthesiologists was associated with significantly higher rates of unexpected hospital admissions compared with that provided by solo anesthesiologists or a care team of physician and CRNA. Who will perform the procedure? Will it be a physician, physician’s assistant, or CRNA who may require physician supervision? Do the recovery room personnel have postanesthesia care experience commensurate with the type of anesthesia anticipated? Will they be able to handle possible complications from the procedure or the anesthetic? In summary, the staffing for all procedures should be adequate to meet the needs of the patient and the providers.
Initially, ambulatory procedures were restricted to those associated with minimal blood loss that could be performed in less than 90 minutes with simple equipment, requiring minimal postoperative care and producing only mild pain that could be controlled with oral medications. Now the only criterion strictly applied is that the patient is able to go home the same day of the procedure, although for some patients a 23-hour hospital stay or other nursing care environment is necessary.
The most complex variable is the patient, whose comorbidities determine whether a procedure may be performed in an ambulatory facility. In 1940, a classification scheme was created to standardize and define the operative risk of patients based on the history and physical examination. Over the past 70 years, this original classification has been modified, with the current ASA physical status (PS) classification divided into 6 categories ( Box 1 ). Although the ASA classification was not created originally as a predictive index of perioperative risk, it has been used as a proxy for risk in several studies.
- 1.
A normal healthy patient.
- 2.
A patient with mild systemic disease.
- 3.
A patient with severe systemic disease.
- 4.
A patient with severe systemic disease that is a constant threat to life.
- 5.
A moribund patient who is not expected to survive without the operation.
- 6.
A patient who has been declared brain-dead and whose organs are being removed for donor purposes.
Overall surgical mortality for patients with ASA PS 1 through 3 is low ( Table 1 ), but as PS class increases, so does the risk for morbidity. In a prospective analysis of 38,598 patients undergoing 45,090 ambulatory procedures, patients with ASA PS 3 constituted 24% of the morbidity. In another study, an ASA PS rating of 2 or 3 predicted a 2-fold greater risk for unanticipated hospital admissions after ambulatory surgery. A retrospective study of 28,921 patients undergoing ambulatory surgery found no significant difference in unplanned admissions between those with ASA PS 3 and those with ASA PS 1 and 2, although those with ASA PS 3 experienced more pain than patients with ASA PS 1 and 2. Although patients with an ASA PS 1 through 3 have low risk with low rates (<2%) of postoperative complications, the overall medical condition of a patient is the most important consideration, and ASA PS alone should not determine eligibility for ambulatory surgery.
| ASA Physical Status Level | 30-Day Mortality Rate (%) |
|---|---|
| 1 | 0.0 ± 0.0 |
| 2 | 0.2 ± 0.1 |
| 3 | 2.2 ± 0.4 |
| 4 | 15.2 ± 2.4 |
| 5 | 70.0 ± 10.5 |
Cardiovascular disease
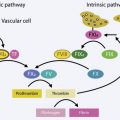
The prevalence of coronary heart disease (CHD) in the United States is now 6%, with the greatest incidence in people 65 years of age or older (19.8%), followed by people aged 45 to 64 years (4.6%). The overall mortality rates for patients with CHD have decreased since the 1960s, and this is attributable to improved medical treatment. In 2007, the American College of Cardiology (ACC) and the American Heart Association (AHA) published updated guidelines for cardiac evaluation and care for patients undergoing noncardiac surgery ( Fig. 1 ). For patients with unstable coronary syndromes, such as unstable or severe angina or recent myocardial infarction, decompensated heart failure, arrhythmias (high-grade or Mobitz II atrioventricular block, third-degree atrioventricular block, symptomatic ventricular arrhythmia, supraventricular arrhythmias with uncontrolled ventricular rate, symptomatic bradycardia, and newly recognized ventricular tachycardia), or severe valvular disease (severe aortic stenosis and symptomatic mitral stenosis), elective surgery should be delayed until further evaluation. In a 1978 study of patients who had a myocardial infarction within 6 months before surgery, 27.3% experienced a perioperative infarction or cardiac death. A 2012 study of the risk of perioperative myocardial infarction in 971,455 patients who had an infarction before surgery demonstrated that only 2% experienced a reinfarction. Despite the apparent dramatic decrease in reinfarction, active cardiac symptoms do signal a risk. For some patients, ambulatory surgery may not be a low-risk procedure.
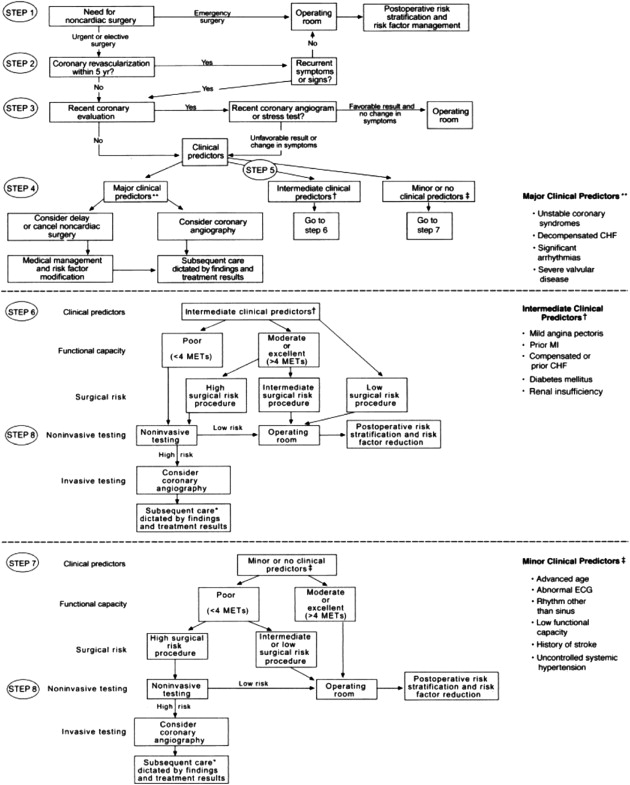
A patient’s functional status is the last criterion to be evaluated under the ACC/AHA algorithm. One metabolic equivalent (MET), the amount of oxygen consumed while sitting at rest, is equal to 3.5 mL of oxygen per kilogram of body weight per minute. The threshold for good functional capacity is 4 METs (able to climb 1 flight of stairs or perform light housework). Patients who were not able to perform 4 METs during daily activities had increased perioperative and long-term risks, and an inverse relationship was seen between the number of blocks or flights a patient could walk and perioperative cardiovascular events. If a patient does not have good functional capacity or is unable to perform 4 METs during daily activities, clinical predictors (eg, ischemic heart disease, heart failure, cerebrovascular disease, diabetes, renal insufficiency) and surgical risk will determine whether further perioperative testing is needed.
For patients with cardiac stents, the ACC, AHA, and the American College of Surgeons have developed joint advisory recommendations for dual antiplatelet therapy. Patients with a bare metal stent should be treated with clopidogrel, 75 mg, and aspirin, 325 mg, for a minimum of 1 month, a sirolimus drug-eluting stent (DES) for a minimum of 3 months, and a paclitaxel DES for a minimum of 6 to 12 months to prevent stent thrombosis. In a large observational study in which antiplatelet therapy was discontinued prematurely for patients with a DES, 29% of patients developed stent thrombosis. Surgery is not recommended for at least 90 days after implantation of a bare metal stent. The odds ratio (OR) for a major cardiac event after surgery within 30 days of stent placement was 3.6, and was 1.6 for surgeries performed between 31 and 90 days of placement. For patients with DES implants, the rate of serious cardiac events was 5.7% to 6.6% for surgeries performed in fewer than 365 days from stent placement and 3.3% in surgeries after 365 days. A cardiologist should be consulted before discontinuation of antiplatelet therapy in these individuals. Patients who are on β-blocker therapy should continue these agents through the perioperative period.
The ACC/AHA guidelines provide the tools to determine whether additional cardiac testing is required before surgery, and following the guidelines for antiplatelet and medical therapy further decreases patient risk. Individuals at high risk for perioperative cardiac events and those with recent myocardial infarctions or cardiac interventions may not be suitable for procedures in an ambulatory setting without access to interventional cardiology.
Cardiovascular disease
The prevalence of coronary heart disease (CHD) in the United States is now 6%, with the greatest incidence in people 65 years of age or older (19.8%), followed by people aged 45 to 64 years (4.6%). The overall mortality rates for patients with CHD have decreased since the 1960s, and this is attributable to improved medical treatment. In 2007, the American College of Cardiology (ACC) and the American Heart Association (AHA) published updated guidelines for cardiac evaluation and care for patients undergoing noncardiac surgery ( Fig. 1 ). For patients with unstable coronary syndromes, such as unstable or severe angina or recent myocardial infarction, decompensated heart failure, arrhythmias (high-grade or Mobitz II atrioventricular block, third-degree atrioventricular block, symptomatic ventricular arrhythmia, supraventricular arrhythmias with uncontrolled ventricular rate, symptomatic bradycardia, and newly recognized ventricular tachycardia), or severe valvular disease (severe aortic stenosis and symptomatic mitral stenosis), elective surgery should be delayed until further evaluation. In a 1978 study of patients who had a myocardial infarction within 6 months before surgery, 27.3% experienced a perioperative infarction or cardiac death. A 2012 study of the risk of perioperative myocardial infarction in 971,455 patients who had an infarction before surgery demonstrated that only 2% experienced a reinfarction. Despite the apparent dramatic decrease in reinfarction, active cardiac symptoms do signal a risk. For some patients, ambulatory surgery may not be a low-risk procedure.
A patient’s functional status is the last criterion to be evaluated under the ACC/AHA algorithm. One metabolic equivalent (MET), the amount of oxygen consumed while sitting at rest, is equal to 3.5 mL of oxygen per kilogram of body weight per minute. The threshold for good functional capacity is 4 METs (able to climb 1 flight of stairs or perform light housework). Patients who were not able to perform 4 METs during daily activities had increased perioperative and long-term risks, and an inverse relationship was seen between the number of blocks or flights a patient could walk and perioperative cardiovascular events. If a patient does not have good functional capacity or is unable to perform 4 METs during daily activities, clinical predictors (eg, ischemic heart disease, heart failure, cerebrovascular disease, diabetes, renal insufficiency) and surgical risk will determine whether further perioperative testing is needed.
For patients with cardiac stents, the ACC, AHA, and the American College of Surgeons have developed joint advisory recommendations for dual antiplatelet therapy. Patients with a bare metal stent should be treated with clopidogrel, 75 mg, and aspirin, 325 mg, for a minimum of 1 month, a sirolimus drug-eluting stent (DES) for a minimum of 3 months, and a paclitaxel DES for a minimum of 6 to 12 months to prevent stent thrombosis. In a large observational study in which antiplatelet therapy was discontinued prematurely for patients with a DES, 29% of patients developed stent thrombosis. Surgery is not recommended for at least 90 days after implantation of a bare metal stent. The odds ratio (OR) for a major cardiac event after surgery within 30 days of stent placement was 3.6, and was 1.6 for surgeries performed between 31 and 90 days of placement. For patients with DES implants, the rate of serious cardiac events was 5.7% to 6.6% for surgeries performed in fewer than 365 days from stent placement and 3.3% in surgeries after 365 days. A cardiologist should be consulted before discontinuation of antiplatelet therapy in these individuals. Patients who are on β-blocker therapy should continue these agents through the perioperative period.
The ACC/AHA guidelines provide the tools to determine whether additional cardiac testing is required before surgery, and following the guidelines for antiplatelet and medical therapy further decreases patient risk. Individuals at high risk for perioperative cardiac events and those with recent myocardial infarctions or cardiac interventions may not be suitable for procedures in an ambulatory setting without access to interventional cardiology.
Pulmonary risk factors
Asthma affects 24.6 million people in the United States, or approximately 8.2% of the population. Chronic obstructive pulmonary disease (COPD) is identified in at least 10 million adults, and the Centers for Disease Control and Prevention (CDC) believes it is underdiagnosed. Although postoperative pulmonary complications are as prevalent as perioperative cardiac complications, pulmonary risk stratification has only recently received attention.
Postoperative pulmonary complications increase length of hospital stay, morbidity, and mortality, and in the case of ambulatory surgery, unplanned admissions. In one study of morbidity and mortality within 1 month of ambulatory surgery, respiratory failure constituted 16% of all morbidity. In a respiratory risk index developed for respiratory failure after vascular or general surgery, respiratory failure was defined as postoperative mechanical ventilation for longer than 48 hours or unanticipated reintubation. Twenty-eight variables were independently associated with respiratory failure, including alcohol use (>2 drinks per day for 2 weeks before the procedure), greater than 10% weight loss in the previous 6 months, elevated blood urea nitrogen levels, low albumin levels, smoking, general anesthesia, and a surgical time from 2.5 to 4 hours. The site and complexity of the operation are the most important factors for evaluating risk of respiratory failure. For procedures with relative value units (RVUs) from 10 to 17, such as orthopedic surgery, exploratory laparotomies, or hysterectomy, the OR for respiratory failure is 2.299 (confidence interval [CI], 1.937–2.728); for RVUs greater than 17 (eg, cardiac surgery, airway surgery, craniotomies), the OR is 4.445 (CI, 3.720–5.312). In other words, patients who undergo cardiac, thoracic, major vascular, or upper abdominal surgery, or head and neck procedures are at increased risk for postoperative respiratory failure.
COPD is the most frequently identified risk factor for postoperative pulmonary complications, such as atelectasis, pneumonia, respiratory failure, and exacerbation of underlying chronic lung disease. In an analysis of 15 studies, the OR was 1.79 (CI, 1.44–2.22) for pulmonary complications in patients with COPD. Another study calculated an OR of 1.517 (CI, 1.362–1.689) for respiratory complications in patients with COPD, and a Canadian study showed that COPD increased operative events by a factor of 2. Patients are at highest risk in the immediate postoperative period from respiratory motor dysfunction, hypoxia, and hypoventilation, and should be closely monitored. COPD in isolation is only a minor risk factor for postoperative respiratory failure.
Whether spirometry or chest radiographs help with pulmonary risk stratification or provide incrementally more information than the history and physical examination has not been determined, and these tests should not be performed routinely for preoperative assessment. Guidelines from the American College of Physicians recommend that patients with stable COPD, irrespective of forced expiratory volume in the first second of expiration (FEV 1 ), be treated with inhaled bronchodilators. Patients with symptomatic COPD and an FEV 1 less than 60% of predicted percentage should be treated with either a long-acting inhaled anticholinergic or a long-acting inhaled β-agonist and treatment should continue through the perioperative period. As for intraoperative management, a meta-analysis showed that spinal and epidural anesthetics decrease postoperative mortality, deep vein thrombosis, pneumonia, and respiratory depression.
Even though the prevalence of asthma in the United States is increasing, morbidity and mortality are decreasing because of advances in medical management. Patients with asthma have varying degrees of airway obstruction, inflammation, and hyperresponsiveness. During anesthesia, aspiration, infection, instrumentation of the airway, the administration of certain drugs, or an inadequate depth of anesthesia may induce bronchospasm, and patients with a history of asthma have a 5-fold risk of postoperative respiratory events. Although perioperative bronchospasm is a concern, it occurs in only approximately 2% of patients. As with patients with COPD, preoperative optimization is critical. Current guidelines indicate that inhaled corticosteroids are the most effective agents for long-term control of the disease. Smoking cessation is a preoperative measure that can decrease the hyperreactivity of the airway, and not smoking for as few as 4 weeks preoperatively improves outcomes. The American Thoracic Society and the European Respiratory Society recommend that patients cease smoking 6 to 8 weeks before surgery.
Pulmonary risk factors alone do not predict postoperative pulmonary complications; the type of procedure and the surgical location are the most important predictors. COPD is not an absolute contraindication to any surgery, but elective surgery should be postponed to treat an exacerbation of COPD or asthma. Well-controlled asthma does not increase risk for perioperative complications.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree