12 Pathophysiology of Stress Urinary Incontinence
ELEMENTS OF URINARY CONTINENCE
Muscular and Supportive Tissue Elements
URETHRA
Urethral Support.
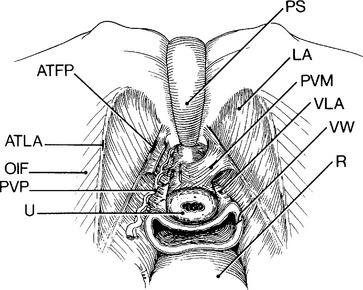
Anterior vaginal wall support directly impacts urethral support (except for lateral attachments of the urethra to the levator ani [pubourethral ligaments]) as the urethra rests on the anterior vaginal wall. The anterior vaginal wall is attached to the arcus tendineus fasciae pelvis (ATFP) that is a condensation of levator ani fascia arising from the surface of the levator ani muscles. The arcus tendineus levator ani is the point of attachment between the levator ani and the obturator internus muscles and lies just cranial to the ATFP. This relationship between the urethra, vagina, and levator ani is a reoccurring theme in understanding the mechanisms of female urinary continence.
Work by Jeffcoate and Roberts (1952), Hodgkinson (1953), and others emphasized the need for intact support of the bladder neck and proximal urethra in a retropubic position for maintenance of urinary continence under stress. This idea highlights urethral support by the anterior vaginal wall that can be altered by defects in vaginal attachments to the levator ani at the ATFP. A stable suburethral layer of anterior vaginal wall prevents urethral and bladder neck descent leading to urethral compression with straining. The emphasis on a retropubic urethra for urinary continence grew out of the success of retropubic operations (i.e., Marshall-Marchetti-Krantz urethropexy) to correct urinary incontinence. The concept is somewhat challenged, however, by the success of midurethral sling procedures that do not ordinarily maintain a retropubic urethra. DeLancey’s (1994) emphasis on the importance of a stable suburethral layer for effective urethral closure over the necessity of a retropubic urethra is perhaps borne from the efficacy of midurethral sling procedures.
The pubourethral ligaments are lateral fascial and muscular attachments of the urethra to the levator ani. These structures, which are supportive of the female urethra and may contribute to urinary continence, are to be distinguished from the pubovesical muscles that probably play a role in voiding by opening the bladder neck. Pubovesical muscles can be seen during a retropubic dissection arching from the ATFP to insert on the urethra (Fig. 12-1); they are easily confused with the pubourethral ligaments that lie beneath. Some speculate that the pubourethral ligaments arise at the midurethra and may augment suburethral supports in promoting urethral compression with strain.
Urethral Coaptation.
The urethra is a pliable structure whose lumen must be completely sealed or coapted to maintain continence. The urethral wall must be sufficiently soft so that external forces can act on it to effect closure. Several studies by Zinner et al. (1980), 1983), using mechanical models, showed higher resistance to water flow when a softer lumen and a lubricating filler were used within the outflow tube. This finding makes clinical sense because a rigid urethra has poor closure properties, as can result from multiple surgeries or radiation. Because clinical scientific studies rarely address this issue, however, the actual importance of urethral softness and mucosal seal, as they pertain to continence, remains uncertain.
LEVATOR ANI
Recognition that the levator ani muscles can be made stronger with pelvic floor exercises and thereby improve female urinary continence has been known since before the 1950s. The levator ani muscles include the pubococcygeus, iliococcygeus, and puborectalis muscles. Of interest in urinary continence is the pubococcygeus muscle that, with contraction, pulls the pelvic floor tightly up and into the pelvic cavity (Fig. 12-2). This action renders a firm backboard for the urethra, particularly in light of the attachments between the urethra (pubourethral ligament), vagina (ATFP), and the levator ani. As discussed in Chapters 2 and 3, innervation of the levator ani does not come from the pudendal nerve. The nerve to the levator ani arises separately from S2–S4 and travels along the medial aspect of the levator muscles. Thus, a woman can possibly have functional levator ani and a dysfunctional urethral sphincter.
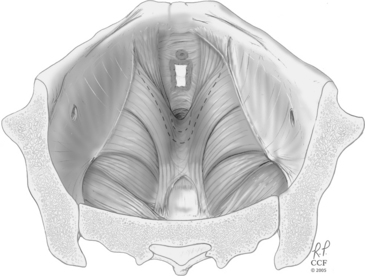
Figure 12-2 Levator ani muscles in a contracted state, narrowing the pelvic opening and supporting the pelvic organs.
(Reprinted with the permission of The Cleveland Clinic Foundation.)
The levator ani and periurethral striated muscles (rhabdosphincter, compressor urethrae, and urethrovaginal sphincter) have a dual role in maintaining urinary continence—they provide resting urethral tone and assist in support (slow-twitch fibers), and they contract rapidly with increased intra-abdominal pressure (fast-twitch fibers). The integration of these two somatic muscle groups is vital to normal urinary continence. During rapid increases in intra-abdominal pressure and with interruption of urination, voluntary and reflex periurethral striated muscle contraction occurs, predominantly in the midurethra and distal urethra, augmenting urethral pressure. Constantinou and Govan (1982) demonstrated that urethral pressure spikes precede, and are often greater than, intravesical pressure spikes during coughing in continent women. Using cinefluorography, Lund et al. (1959) observed two actions when a woman is asked to interrupt her urine stream. The first is a prompt constriction of the voluntary musculature that immediately interrupts the urine stream in the midurethra, presumably due to periurethral striated muscle contraction against a stable suburethral base. The urine distal to the constriction is voided, but the contents of the proximal urethra are forced back into the bladder. Simultaneously, the base of the bladder is seen to rise and is drawn cephalad, presumably due to levator contraction acting on levator attachments to the suburethral base and bladder (i.e., anterior vaginal wall). Both actions are characteristic of voluntary fast-twitch muscle contraction; in fact, the diameter of fast-twitch muscle in the levator ani has been correlated with urethral pressures during periods of stress. Emphasizing the role of levator support, Heidler et al. (1987) noted that with sneezing in dogs, pressure increases in the distal urethra decrease after transecting the pelvic muscles from the urethra. Taken together, normal urinary continence in women relies on multiple redundant interconnected mechanisms.
Neurophysiologic Considerations
Chapter 3 describes in detail the neurophysiology of the lower urinary tract. Reexamining those concepts now is important to connect the aforementioned anatomic considerations with their neurologic connections. The disconnect between anatomy and function, which can appear in cases of complex urinary incontinence, perhaps stems from inadequate understanding of the neurologic basis of female continence. Furthermore, the fact that medications can affect stress-related urinary incontinence testifies to the role of nonanatomic elements in female urinary continence.
EFFERENT AND AFFERENT PATHWAYS
The parasympathetic nerves that innervate the detrusor exit the spinal cord between S2 and S4. As with all parasympathetic nerves, the preganglionic neurotransmitter is acetylcholine (ACh), yet the postganglionic neurotransmitter varies with the target. Postganglionic parasympathetic neurotransmission to the urethral smooth muscle is via nitric oxide, while both ACh and adenosine triphosphate (ATP) serve in this role to the detrusor smooth muscle. The contribution of purinergic stimulation of the detrusor is likely minor under normal settings, although up-regulation under certain conditions may contribute to development of an overactive bladder (O’Reilly et al., 2002). The role of nitric oxide in urethral smooth muscle contraction is inhibitory. Parasympathetic efferent stimulation of detrusor smooth muscle cells is mediated by muscarinic receptors, of which there are two: M2 and M3. Although the M2 receptor is the most abundant, the M3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree