13 Obstetrics and Pelvic Floor Disorders
IMPACT OF CHILDBIRTH ON PELVIC MUSCLE AND NERVE FUNCTION
Anatomic Outcomes—Pelvic Floor Muscles
Readers of this chapter have likely witnessed countless times the miracle of childbirth and have also likely considered the “P’s”—that is, how the power pushes the passenger through the passage. In a classic review, Power (1946) described the mechanism by which the fetus negotiates the birth canal and is expelled through the pelvic diaphragm as follows: As the flexed fetal head strikes the pelvic floor, the levator ani muscle segments are funneled from behind and forward. The ischiococcygeus muscle is the first to receive the impact, but the head is often preceded by a dilating wedge of amniotic fluid and membranes that transfers most of the pressure onto the front of the pubococcygeus muscle. The anococcygeal raphe is pushed down until it becomes vertical. The ischiococcygeus assumes a vertical plane and acts as a deflecting surface for the descending head, which is deflected downward and forward onto the iliococcygeus.
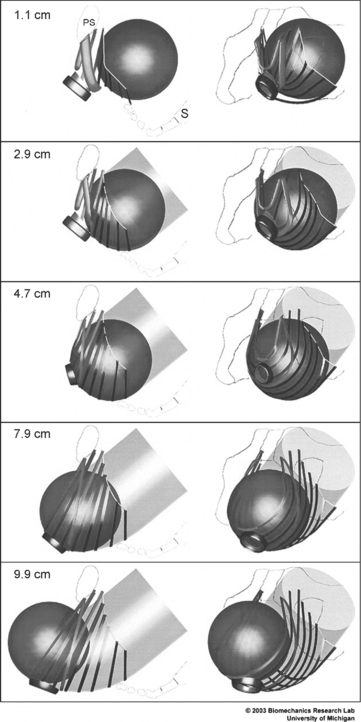
Power (1946) based his description on direct observation, the study of anatomy, and conjecture. Recently, Lien et al. (2004) used sophisticated imaging techniques to develop a biomechanical model to describe changes in levator ani muscles as the fetal head descends through the vagina (Fig. 13-1). The medial pubococcygeus muscle reached an impressive stretch ratio (defined as tissue length under stretch/original tissue length) of 3.26. According to the authors, this exceeds the greatest stretch ratio (1.5) seen in passive striated muscle of nonpregnant women by 217%. Increasing the fetal head diameter by 9% increased medial pubococcygeus stretch by the same proportion. This model suggests that the medial pubococcygeus muscle has the greatest risk for injury of all the levator ani muscles during the second stage of labor. This supposition is supported by magnetic resonance imaging (MRI) studies that reveal abnormalities in this area in 20% of primiparous women after vaginal delivery.
Between 1945 and 1955, Gainey (1955) systematically evaluated 2000 patients and compared the prevalence of postpartum pelvic tissue damage between women who were delivered with no episiotomy and those delivered via mediolateral episiotomy. In this nonrandomized, nonmasked cohort study, he found higher rates of tissue damage in women delivered without episiotomy. Thirty-one percent and 24% of women delivered without episiotomy had levator atrophy and detached rectovaginal septum, respectively, compared with 12% and <1% of women, respectively, in whom a mediolateral episiotomy was made. Episiotomy appeared to protect the patient from pelvic tissue damage; thus some form of episiotomy became the standard in obstetric management for decades. Only with an emerging understanding of the importance of study design and analysis in drawing conclusions did modern obstetricians begin to reject routine “prophylactic” episiotomy.
Anatomic Outcomes—Anal Sphincter Muscle
Endoanal ultrasound performed in the first months postpartum reveals that as many as 35% of primiparous women and up to 44% of multiparous women have evidence of sphincter disruption. Other anal function studies, such as anorectal manometry and anorectal sensation testing, also have demonstrated that vaginal delivery has an effect on anal function. Chaliha et al. (2001) showed that, compared to before delivery, vaginal delivery was associated with decreased anal squeeze pressures and resting pressures, whereas no change in anal sensation was noted. Risk factors for both overt and occult sphincter injuries include forceps, prolonged second stage, large birth weight, midline episiotomy, and occipitoposterior positions. Table 13-1 describes the typical adjusted odds ratios for predictors of anal sphincter disruption on multivariate analyses. In addition, Richter et al. (2002) reported that vaginal birth after cesarean delivery was an independent risk factor for anal sphincter disruption, compared to previous vaginal delivery (relative risk 5.46 [3.69–8.08]) after adjusting for primiparity, birth weight greater than 4000 g, forceps delivery, vacuum delivery, shoulder dystocia, and episiotomy.
Table 13-1 Predictors of Anal Sphincter Disruption After Vaginal Delivery
| Risk Factors | Adjusted Odds Ratios* |
|---|---|
| Midline episiotomy | 4.9–16.5 |
| Nulliparity | 2.5–4.0 |
| Operative delivery | 2.5–3.5 |
| Birthweight ≥4000g | 1.5–2.5 |
| Occipitoposterior | 1.2–1.8 |
* Numbers represent the minimum and maximum adjusted odds ratios reported in the medical literature.
Randomized trials have also supported the association between episiotomy and anal sphincter disruption. Harrison et al. (1984) randomized 181 primigravid women to routine mediolateral episiotomy or to restricted use of mediolateral episiotomy. The episiotomy group sustained rectal injury in 5 of 89 cases (5.6%), compared to no cases of rectal injury in the restricted group. Sleep et al. (1984) randomized 1000 women to liberal use of mediolateral episiotomy (51% of patients) or restricted mediolateral episiotomy (10% of patients). A liberal policy toward episiotomy resulted in significantly more maternal vaginal trauma and more suturing.
The association between operative delivery and perineal trauma has also been studied in a randomized fashion. Yancey et al. (1991) randomly assigned uncomplicated, term gestations at 2+ station in occipitoanterior position to routine outlet forceps or to spontaneous delivery. Among patients delivered by outlet forceps, the incidence of third- or fourth-degree laceration was 30 of 165 (18%) versus 12 of 168 (7%) in women who delivered spontaneously. Midline episiotomy and outlet forceps were the only factors significantly associated with rectal trauma on multivariate analysis.
Neurologic Outcomes
Ample evidence links neurologic injury with pelvic floor disorders. It appears that labor and childbirth, but not pregnancy, are major risk factors for neurologic injury. For example, in a study by Sultan et al. (1994), the pudendal nerve terminal motor latency (PNTML) was not increased in pregnant non-laboring women compared with non-pregnant women. However, after vaginal delivery, Snooks et al. (1986) found that 42% of women had a prolonged PNTML, but this had eventually recovered in most women by 2 months postpartum. Single-fiber electromyography (EMG) of the anal sphincter revealed an increased fiber-density 2 months postpartum. These findings were most striking in multiparous women (suggesting a cumulative effect of vaginal delivery on the pelvic floor) and women delivered by forceps. No changes in these parameters were seen in women who had delivered by cesarean section. Five years after delivery, Snooks et al. (1990) reported that there was manometric and neurophysiologic evidence of weakness because of partial denervation of the pelvic floor striated sphincter musculature, with pudendal neuropathy, which was more marked in those women with incontinence. Other investigators have confirmed that latencies are prolonged after vaginal delivery and have also confirmed that the conduction time returns to normal in most women.
In a prospective study by Allen et al. (1990), concentric-needle EMG was performed on the levator ani before delivery as well as 2 and 5 days after delivery. Following vaginal delivery, the duration of the motor unit potential was increased, consistent with nerve damage. Eighty percent of the women showed evidence of denervation with subsequent reinnervation after vaginal delivery. After elective cesarean delivery, however, the motor unit potentials were unchanged. In contrast to the findings after elective cesarean deliveries, the duration of the motor unit potentials was increased following emergency cesarean deliveries that were performed after labor onset. Similarly, other investigators have found the PNTML to be prolonged after a cesarean delivery performed in late labor (cervical dilation 8 cm or greater) but not in women delivered by cesarean before they reached 8 cm dilation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree