The ever-increasing incidence of primary cutaneous malignancies has heralded the need for multiple treatment options. Surgical modalities remain the mainstay of treatment of nonmelanoma skin cancer. However, it is important for the dermatologic surgeon to have an understanding of all treatment options to assist the patient in making the most informed decision possible, ultimately leading to the most favorable outcome. This article explores the available nonsurgical treatment options, their indications, and their efficacy.
Nonmelanoma skin cancer (NMSC) is the most common type of cancer in humans, accounting for more than 1 million new cases in the United States in 2009. The increased incidence of primary cutaneous malignancies has led to the need for multiple treatment options. Surgery remains the mainstay of treatment of NMSC and should be the preferred method of treatment in many circumstances ( Box 1 ). However, it is essential for the dermatologic surgeon to have a thorough understanding of the various nonsurgical modalities ( Box 2 ) for the treatment of NMSC. This information is equally important to patients in order for them to make the most informed decision in the treatment of their malignancy.
Recurrent tumor
Tumors with aggressive histologic features (perineural, deeply invasive, poorly differentiated)
High-risk locations (periorificial, embryonic fusion planes, mucosal)
Tumor arising adjacent to a scar
Traditional nonsurgical modalities
Electrodessication and curettage
Cryosurgery
Radiotherapy
Topical modalities
Imiquimod
5-Fluorouracil (5-FU)
Diclofenac
Retinoids
Newer agents
Ingenol mebutate
Resiquimod
Emerging nonsurgical modalities
Chemical peels
Laser ablation
Intralesional therapy
5-FU
Bleomycin
Interferon-α
Photodynamic therapy
Oral capecitabine
Nonsurgical physical modalities
Electrodessication and Curettage
Electrodessication and curettage (ED&C) is one of the most widely used methods used in the treatment of basal cell carcinoma (BCC), squamous cell carcinoma in situ (SCCIS), and squamous cell carcinoma (SCC). Electrodessication uses markedly damped, high-voltage, low-amperage current to produce superficial tissue destruction. ED&C is performed by first marking an outline of the tumor margins and anesthetizing the area around the lesion with lidocaine with epinephrine. The tumor is then curetted in a checkerboard pattern until the lesion is removed. The lesion is touched with the tip of the electrode until a grayish, superficial charred layer covers the lesion. The charred debris is removed from the treated lesion with a curette. The process is repeated 2 to 3 times until the tumor has been eradicated. The success rate of ED&C is operator dependent because the surgeon must be able to detect subclinical tumor using the curette. Low-risk areas with a thick underlying dermis such as the trunk and extremities are ideal locations for ED&C. The treatment of lesions in these areas usually leads to rapid healing and high cure rates. If the tumor extends into the subcutaneous fat, the procedure should be abandoned and an excision performed. In patients with implantable cardioverter-defibrillators or cardiac pacemakers, electrocautery should be used instead. Proper tumor and patient selections are crucial to achieve complete tumor clearance and to avoid recurrence. Primary tumors with distinct clinical borders located on sites, such as non–H zone regions of the face, trunk, and extremities, or of superficial or nodular histologic subtype with a diameter of less than 1 cm on the face and less than 2 cm on the trunk and extremities can be treated effectively with ED&C. Treatment sites can be divided into low-, medium-, and high-risk areas ( Box 3 ). Several large retrospective studies have shown that the 5-year recurrence rate for primary BCC’s less than 1 cm in diameter treated with ED&C ranges from 3.3% to 5.7%. The 5-year recurrence rates of SCC treated with ED&C are similar. One study found no difference in the recurrence rates of SCC and BCC. A prospective study comparing ED&C with cryotherapy showed a lower recurrence rate with ED&C (12% vs 50%). The ED&C cohort also had shorter healing times (median of 36 days and 46 days, respectively).
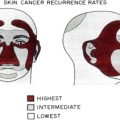
High
Nose
Nasolabial folds
Ear
Chin
Periorificial
Medium
Other facial sites not included in high risk areas
Low
Trunk
Extremities
Risk factors of ED&C include hypopigmentation, hypertrophic scars and keloids, depressed or atrophic scars, and tissue contracture. Cosmetic outcome of ED&C is site dependent. On the face, ED&C usually heals with a thin white plaque that may be depressed or indurated. In general, surgical excision is superior to ED&C when treating lesions on the face because it often leads to a better cosmetic outcome. On areas such as the trunk and extremities, as well as concave areas, ED&C may provide a good cosmetic result. ED&C is an efficient cost-effective procedure that has consistently shown high cure rates and should be considered as an alternative first-line treatment of small primary, nonaggressive BCC and SCC.
Cryosurgery
Cryosurgery is a cost-effective and versatile modality used in the treatment of benign, premalignant, and malignant skin lesions. A cryogen, most commonly liquid nitrogen (boiling point −195.6°C), is applied to the skin using a handheld device using an open spray technique or probe. Tissue injury is caused by 2 mechanisms: ice crystal formation within the cells and vascular thrombosis during freezing followed by vascular stasis after thawing. This injury ultimately leads to ischemic necrosis of the tissue. A pyrometer-thermocouple device may be used to monitor tissue temperature and depth of freezing. A final tissue temperature of −50°C to −60°C is needed for destruction of malignant lesions. Pulsing, or intermittent spray technique, is recommended when treating malignant lesions. Using this method, the “ice ball” will have a greater depth while minimizing lateral spread. The maximum depth of penetration is 10 mm. On the contrary, when treating superficial lesions, a continuous spray technique can be used to limit the depth of penetration. Premalignant lesions such as actinic keratoses (AKs) usually require 1 or 2 freeze-thaw cycles of 5 to 7 seconds with a lateral spread to the edge of the lesion. When treating malignant tumors, the goal is to destroy the same amount of tissue that would be removed during an excision of the same lesion. Therefore, it is essential that an adequate margin be frozen around the area of the tumor. The lateral spread should form a 3- to 5-mm halo around the lesion. Malignant tumors require 2 to 3 freeze-thaw cycles. Larger lesions should be curetted and debulked before freezing, which enables the lesion to be frozen more rapidly. The halo time, or the duration of thawing of the marginal surface around the lesion, should be longer than 60 seconds. The total thaw time for the lesion should exceed 90 seconds. Although cryosurgery is an efficient and effective treatment option in many cases, cryosurgery is not recommended for treatment of ill-defined lesions, tumors with an aggressive histologic subtype, or lesions that are deeply invasive.
Common postoperative symptoms include pain, swelling, tenderness, and redness. Blisters and crusting may occur when treating malignant lesions with longer freeze times with deep depth of penetration. Patients can be given a sterile 22-gauge needle to drain the blister or bullae. Complications of cryosurgery include hypopigmentation or hyperpigmentation, blister or bullae formation, nerve damage, and secondary infection. Caution must be taken not to damage surrounding structures, such as hair follicles (alopecia), superficial cutaneous nerves (dysesthesia), nail matrix (onychodystrophy), and cartilage (notching of the nose or ear). Occasionally, hypertrophic and keloid scars may form. Cosmetically, cryosurgery is comparable to radiotherapy but worse than photodynamic therapy (PDT) and surgical excision. In studies comparing cryotherapy with PDT, cosmetic outcomes were rated as good or excellent in 89% to 94% of patients treated with PDT versus 50% to 66% in patients treated with cryotherapy. In a prospective study comparing cosmetic outcomes of surgical excision and cryosurgery, both patients and clinical professionals preferred surgical excision to cryosurgery. In addition, cryosurgery may be associated with longer healing times and more discomfort than other nonsurgical modalities. With superior cosmesis and lower recurrence rates, surgical excision remains the treatment of choice when practical.
Radiotherapy
Radiation therapy is a valuable alternative to surgical management of SCC and BCC, especially in patients with inoperable tumors and in those with significant comorbidities who would have a difficult time undergoing an extensive surgery. For further discussion of radiotherapy for the treatment of NMSC, see article by Hulyalkar and colleagues elsewhere in this issue.
Topical modalities
Many topical treatments have been used as both monotherapy and adjuvant methods to treat NMSC (see Box 2 ). Advantages of topical regimens include excellent cosmesis with less risk of scarring, convenience, and ability to treat large areas. Potential disadvantages include cost of the agents and patient compliance, especially in patients requiring long treatment duration. One must also be aware that treatment with topical therapy could potentially cause skip lesions and subsequently lead to false-negative margin findings that theoretically reduces the efficacy of surgical excision and Mohs micrographic surgery. In addition, results may be delayed, and patients may become discouraged and discontinue the medication.
Imiquimod
Imiquimod 5% cream is an immunomodulating agent that is Food and Drug Administration (FDA)-approved for topical use in the treatment of AKs and superficial BCC in immunocompetent adults. Imiquimod 3.75% has recently been approved by the FDA and can be used daily on larger areas of skin, the balding scalp, or the full face. In contrast, imiquimod 5% is indicated for use on areas of skin that are 25 cm 2 or smaller. Imiquimod has been used effectively (with varying treatment regimens) in the treatment of AKs, superficial BCC, nodular BCC, and SCCIS ( Table 1 ). Imiquimod works by stimulating both innate and cell-mediated immune responses and has antiviral, antitumoral, and immunoregulatory properties. Antitumoral mechanisms of action of imiquimod are listed in Box 4 .
| Tumor Type | Dosing | Treatment Duration | Results | References |
|---|---|---|---|---|
| AKs | 5% cream qhs 2–3 times weekly | 12–16 wk | 50% complete and 75% partial clearance | Hadley et al |
| 2.50% and 3.75% cream | 2 wk on/off/on × 8 wk | Complete and partial clearance of 30.6% and 48.1% for imiquimod 2.5% and 35.6% and 59.4% for imiquimod 3.75%, respectively | Swanson et al | |
| Superficial BCC | 5% cream qhs 3–7 times weekly | 6–16 wk | 50%–100%, >90% 5–7 times weekly | Marks et al, Geisse et al |
| 80% clearance at 5 years | Quirk et al | |||
| Nodular BCC | 5% cream qhs under occlusion 7d/wk | 6–12 wk | 70%–100% clearance, greatest efficacy at 12 wk | Huber et al, Shumack et al |
| SCCIS | 5% cream qd-qod | 6–16 wk | 90%–100% clearance | Mackenzie-Wood et al |
| SCC | 5% cream 5–7 times per wk | 12 wk | Limited follow-up | Martin-Garcia |
Induced production of cytokines, including interferon (IFN)-α, tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-2, IL-6, IL-8, and IL-12, by human peripheral blood mononuclear cells
Stimulation of monocytes, macrophages, and toll-like receptor (TLR)-7– and TLR-8–bearing plasmacytoid dendritic cells, and epidermal Langerhans cells. Stimulation of TLRs induces the production of proinflammatory cytokines involved in the innate immune system
Production of IL-6, IL-8, and IFN-α by keratinocytes, leading to a T H 1-dominant response
Increase in type I IFN, improving the response to endogenous IFN-α, usually low in AKs
Suppression of type I IFN signaling proteins, which is an early event leading to SCC
Induction of FasR (CD95), a member of the TNF receptor family, involved in apoptosis
Induction of proapoptotic pathways associated with B-cell lymphoma/leukemia 2 (Bcl-2)–associated X (Bax) protein
Induction of caspases 3 and 9, which have been linked to stress signaling, mitochondrial death pathways, and apoptosis
Induction of E-selectin (a ligand for lymphocyte antigen expressed by skin-resident T cells that are responsible for immunosurveillance) on blood vessels of invasive SCCs, which is usually absent in SCCs
Reduction of T-regulatory cells, which express FOXP3, infiltrating SCCs. These T-regulatory cells cause impairment of effector T cells (responsible for immunosurveillance), surround the tumor, and prevent cells from reaching the tumor
Imiquimod should be applied sparingly to focal areas when treating superficial BCC. A broader treatment area is recommended either on the scalp or face, but not both, when treating AKs. Imiquimod should be applied before bedtime and should be left on the skin for 6 to 10 hours. Imiquimod is generally well tolerated, and irritant reactions can be treated by temporary discontinuation of therapy and application of topical steroids. Therapy can be resumed as necessary at a decreased frequency as indicated. Erythema involving the area of application is seen in most patients treated with imiquimod. Pruritus, burning sensation, stinging sensation, and tenderness are other common side effects. Other reported adverse events include hypopigmentation, fever, diarrhea, and fatigue.
AK
Imiquimod is FDA approved for the treatment of AKs involving the head and neck. Many treatment regimens have been used in treating AKs with varying degrees of success. A meta-analysis and systemic review evaluating efficacy and toxicity in the treatment of AKs concluded that imiquimod 5% cream 2 to 3 times weekly for 12 to 16 weeks resulted in complete clearing in 50% of patients (partial clearing of 75%) versus 5% of those treated with placebo. Toxicity was limited to irritant contact dermatitis and was well managed with temporary cessation of the drug.
Superficial BCC
Imiquimod 5% cream is FDA approved for the treatment of superficial BCCs less than 2 cm in diameter on the neck, trunk, and extremities. Optimal dosing consists of nightly application 5 to 7 times a week for 6 to 16 weeks (depending on the patient’s clinical response). Cure rates have been shown to be greater than 90%.
Nodular BCC
Treatment responses to imiquimod for nodular BCC are not as high as those seen in superficial BCC. In two phase 2 studies, the highest response rates achieved using imiquimod 5% cream twice daily for 12 weeks was around 75%. Therefore, this modality should be limited to patients with small tumors in low-risk areas who cannot undergo surgery or receive radiation therapy or cryotherapy.
SCCIS
SCCIS, or Bowen disease, responds to treatment with imiquimod 5% cream. In an open-label study of 16 patients with SCCIS of the lower extremities, a 93% cure rate was attained using imiquimod 5% cream once daily for up to 16 weeks.
SCC
Imiquimod is not recommended for the treatment of invasive SCC. In a case report and case series, Marin-Garcia achieved optimal results with nightly applications 5 to 7 times weekly for 12 weeks. However, follow-up of these patients was limited. Therefore, imiquimod may be a viable treatment option in patients who refuse surgery or are not good surgical candidates.
5-Fluorouracil
5-Fluorouracil (5-FU) is a structural analogue of thymine that inhibits thymidylate synthetase, a critical enzyme in DNA synthesis. 5-FU prevents cellular proliferation, ultimately resulting in cell death. The effects are most pronounced in rapidly dividing cells. 5-FU has been used to treat various malignancies, including breast cancer, colorectal cancer, and tumors of the head and neck. The use of 5-FU as a topical agent began after a case report describing resolution of AKs in a patient receiving systemic 5-FU.
In 1963, a hydrophilic ointment of 20% 5-FU was initially used in the treatment of patients with diffuse AKs for 4 weeks. Further studies testing multiple concentrations of 5-FU showed that the 5% ointment was comparable with the 20% ointment. Since then, 5-FU has been used to treat multiple skin conditions, including extensive AKs, SCCIS, actinic cheilitis, porokeratosis, and verruca vulgaris.
The principal indication of 5-FU is for the treatment of AKs, with twice daily dosing for 1 month. 5-FU is a practical alternative to treating large areas with extensive actinic damage on the face and neck without damaging normal skin. 5-FU has also been used successfully in the treatment of superficial BCC, actinic cheilitis, and Bowen disease, especially when used in conjunction with other modalities such as ED&C.
The standard of care for the treatment of AKs has been with 5% 5-FU. More recently, 1.0% and 0.5% cream formulations have become available. One study showed the 0.5% formulation to be as efficacious as and better tolerated than the conventional 5% formulation. The 0.5% formulation contains 5-FU in a patented porous microsphere delivery system that delivers a therapeutic dose of 5-FU while minimizing systemic absorption. 5-FU is commercially available as 1%, 2%, and 5% solutions and 0.5%, 1%, and 5% creams. The 1%, 2%, and 5% formulations should be applied twice daily, whereas the 0.5% formulation is to be used once daily. Treatment durations range from 2 to 6 weeks depending on the site treated and the patient’s response and tolerability of the treatment. Patients should be instructed to avoid excessive application and to avoid sensitive areas such as the oral commissure, nasolabial creases, and eyes.
Topical 5-FU should be avoided in patients with known allergies to 5-FU and in patients who have known deficiency of dihydropyrimidine dehydrogenase (DPD), an enzyme critical in the metabolism of 5-FU.
Erythema, irritation, burning sensation, pruritus, pain, hypopigmentation, and hyperpigmentation are the most common adverse effects. Symptoms are expected within the initial 5 to 10 days of treatment. Allergic contact dermatitis caused by 5-FU has also been reported. An inflammatory reaction is expected, including crusting, edema, and oozing. Frequency of application or strength of 5-FU may be reduced in patients who experience severe reactions. Topical steroids may be used concomitantly and 1 to 2 weeks after treatment with topical 5-FU to reduce inflammation.
Diclofenac
Diclofenac is a nonsteroidal antiinflammatory drug that is a potent inhibitor of inducible cyclooxygenase (COX) 2, resulting in a reduction of prostaglandin synthesis. COX is the rate-limiting enzyme in the synthesis of prostaglandins from arachidonic acid. Increased production of prostaglandins may be associated with the development of NMSC. Prostaglandin E2 plays a role in Bcl-2 gene expression, IL-6 production, and inhibition of apoptotic pathways. COX-2 has been shown to be overexpressed in a variety of different malignancies, including colon carcinoma, SCC of the esophagus, and skin cancers (AKs, melanoma, and NMSC). It is thought that by inhibiting COX-2, tumor growth can be slowed via inhibition of angiogenesis, apoptosis, and tumor invasion. By the same principle, multiple UV-induced proinflammatory cytokines, such as IL-1, TNF-α, and transforming growth factor β also capable of inducing COX-2, may be inhibited by diclofenac. Hyaluronic acid 2.5% vehicle gel decreases the diffusion of diclofenac through the skin and subsequently increases the contact time with the epidermis, thereby increasing uptake of diclofenac by the atypical cells. Topical diclofenac 3% gel has been used successfully in the treatment of AKs and is usually well tolerated. In one study, twice-daily application for 60 to 90 days was shown to decrease the number of AKs by 64%, and 33% of patients had complete clearance.
Retinoids
All-trans retinoic acid (tretinoin) was the first topical retinoid studied and found to be beneficial in the treatment of AKs. Tretinoin has been shown to reduce the number of AKs on facial skin by up to 50% when used as monotherapy over a period of 6 months. However, there was no significant improvement of lesions on the scalp and extremities. Tretinoin may also be used in combination with 5-FU in the treatment of AKs. Use of tretinoin leads to enhanced penetration of 5-FU and enhanced efficacy.
Tazarotene is a prodrug that is hydrolyzed in the tissue to form the active metabolite, tazaratenic acid. Tazarotenic acid has a high affinity for the retinoic acid receptor γ nuclear receptor in the epidermis. By binding to this receptor, cell proliferation and differentiation is regulated. A few small studies have shown tazarotene to be an effective treatment of NMSC. Peris and colleagues achieved 53% clearance, 47% decreased size, and no tumor progression in a cohort of patients treated for BCC with 0.1% tazarotene gel once daily for 5 to 8 months.
Side effects include erythema, burning sensation, and pruritus. Tazarotene is a pregnancy category X medication and should be avoided in pregnant patients, whereas tretinoin is a pregnancy category C medication. Caution must be taken in patients who continue to have significant sun exposure.
The use of systemic retinoids in high-risk patient populations has been shown to be effective in the chemoprevention of NMSC. An in-depth discussion of systemic retinoids in organ transplant patients may be found in an article elsewhere in this issue.
Newer Topical Agents
Ingenol mebutate
Ingenol mebutate (IM) is an extract from the plant Euphorbia peplus (milkweed), which has been used for several years as a home remedy treatment of skin conditions, including AKs and skin cancers. IM is thought to work by disrupting the plasma membrane, leading to mitochondrial swelling of dysplastic keratinocytes and subsequent cell death. IM also promotes healing and restoration of the normal clinical and histologic morphology. Further generation of tumor-specific antibodies, along with proinflammatory cytokines and neutrophil infiltration, results in antibody-dependent cellular cytotoxicity that eliminates residual cells. IM comes in formulations of 0.025% and 0.050%. Several controlled studies have shown IM to be efficacious in the treatment of AKs. IM is generally well tolerated. Adverse effects include mild erythema, scaling, and crusting.
Resiquimod
Resiquimod is a TLR 7/8 agonist that has comparable stimulatory effects on monocytic cells, although it is 10- to 100-times more potent than imiquimod. In addition, resiquimod induces IL-1 receptor antagonist, granulocyte/macrophage colony-stimulating factor, granulocyte colony-stimulating factor, macrophage inflammatory protein, macrophage inflammatory protein 1β, inflammatory protein 1α, and monocyte chemotactic protein. One phase 2 study consisting of 132 patients showed an efficacy of 40.0% to 74.2% in the treatment of AKs when treating 4 to 8 lesions on the scalp with various concentrations of resiquimod 3 times weekly. There was no difference in efficacy among the various concentrations, but lower concentrations produced better tolerability.
Topical modalities
Many topical treatments have been used as both monotherapy and adjuvant methods to treat NMSC (see Box 2 ). Advantages of topical regimens include excellent cosmesis with less risk of scarring, convenience, and ability to treat large areas. Potential disadvantages include cost of the agents and patient compliance, especially in patients requiring long treatment duration. One must also be aware that treatment with topical therapy could potentially cause skip lesions and subsequently lead to false-negative margin findings that theoretically reduces the efficacy of surgical excision and Mohs micrographic surgery. In addition, results may be delayed, and patients may become discouraged and discontinue the medication.
Imiquimod
Imiquimod 5% cream is an immunomodulating agent that is Food and Drug Administration (FDA)-approved for topical use in the treatment of AKs and superficial BCC in immunocompetent adults. Imiquimod 3.75% has recently been approved by the FDA and can be used daily on larger areas of skin, the balding scalp, or the full face. In contrast, imiquimod 5% is indicated for use on areas of skin that are 25 cm 2 or smaller. Imiquimod has been used effectively (with varying treatment regimens) in the treatment of AKs, superficial BCC, nodular BCC, and SCCIS ( Table 1 ). Imiquimod works by stimulating both innate and cell-mediated immune responses and has antiviral, antitumoral, and immunoregulatory properties. Antitumoral mechanisms of action of imiquimod are listed in Box 4 .
| Tumor Type | Dosing | Treatment Duration | Results | References |
|---|---|---|---|---|
| AKs | 5% cream qhs 2–3 times weekly | 12–16 wk | 50% complete and 75% partial clearance | Hadley et al |
| 2.50% and 3.75% cream | 2 wk on/off/on × 8 wk | Complete and partial clearance of 30.6% and 48.1% for imiquimod 2.5% and 35.6% and 59.4% for imiquimod 3.75%, respectively | Swanson et al | |
| Superficial BCC | 5% cream qhs 3–7 times weekly | 6–16 wk | 50%–100%, >90% 5–7 times weekly | Marks et al, Geisse et al |
| 80% clearance at 5 years | Quirk et al | |||
| Nodular BCC | 5% cream qhs under occlusion 7d/wk | 6–12 wk | 70%–100% clearance, greatest efficacy at 12 wk | Huber et al, Shumack et al |
| SCCIS | 5% cream qd-qod | 6–16 wk | 90%–100% clearance | Mackenzie-Wood et al |
| SCC | 5% cream 5–7 times per wk | 12 wk | Limited follow-up | Martin-Garcia |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree