CHAPTER 5 Noninvasive Examination of the Patient Before Sclerotherapy
Confirmatory diagnostic testing is performed after the history is taken and the physical examination performed. The handheld continuous-wave Doppler historically was a routine part of the physical examination but is slowly being replaced by compact, portable duplex ultrasound visualization. At present, the vascular laboratory (level 2) provides a reliable tool for acquiring anatomic and functional information that not only confirms the diagnosis but also formulates treatment. Elements of the diagnostic vascular laboratory are slowly becoming part of a routine physical examination.1 A duplex examination of the venous system is becoming a more important diagnostic tool. This approach has been confirmed in the guidelines of the American Venous Forum.2
Medical History
CEAP classification
An international ad hoc committee of the American Venous Forum developed the CEAP classification for chronic venous disease in 1994 (Table 5.1; see also Chapter 2) with the goal of stratifying clinical levels of venous insufficiency. The four categories and descriptors selected for classification were clinical state (C), etiology (E), anatomy (A), and pathophysiology (P). The CEAP classification has been endorsed worldwide, despite its acknowledged deficiencies. It has been adopted as a standard in many clinics in Europe, Asia, South America, and the United States it is considered the only modern method for reporting data. It’s weakness is the inability to distinguish between levels of smaller superficial veins. The CEAP classification was revised in 2004,3 and is now referred to as ‘advanced’ CEAP.4 It includes:
| CLINICAL CLASSIFICATION | |
| C0 | No visible or palpable signs of venous disease |
| C1 | Telangiectasias or reticular veins |
| C2 | Varicose veins – separated from reticular veins by a diameter of 3 mm as the upper limit of size of a reticular vein |
| C3 | Edema |
| C4 | Changes in the skin and subcutaneous tissue secondary to chronic venous disease are divided into two subclasses to better define the differing severity of venous disease: |
| C4a | Pigmentation and eczema |
| C4b | Lipodermatosclerosis and atrophie blanche |
| C5 | Healed venous ulcer |
| C6 | Active venous ulcer |
| Each clinical class is further characterized by a subscript for the presence of symptoms (S, symptomatic) or their absence (A, asymptomatic). Symptoms include aching, pain, tightness, skin irritation, heaviness, and muscle cramps, as well as other complaints attributable to venous dysfunction. | |
| ETIOLOGIC CLASSIFICATION | |
| Ec | Congenital |
| Ep | Primary |
| Es | Secondary (postthrombotic) |
| En | No venous etiology identified |
| ANATOMIC CLASSIFICATION | |
| As | Superficial veins |
| Ap | Perforator veins |
| Ad | Deep veins |
| An | No venous location identified |
| PATHOPHYSIOLOGIC CLASSIFICATION | |
| Basic CEAP | |
| Pr | Reflux |
| Po | Obstruction |
| Pro | Reflux and obstruction |
| Pn | No venous pathophysiology identifiable |
| Advanced CEAP | |
| Same as Basic with the addition that any of 18 named venous segments (below) can be utilized as locators for venous pathology. | |
| All items listed in C should be repeated. | |
| VENOUS SEGMENTS | |
| Superficial veins | |
| 1 | Telangiectasias/reticular veins |
| 2 | GSV above knee |
| 3 | GSV below knee |
| 4 | SSV |
| 5 | Nonsaphenous veins |
| Deep veins | |
| 6 | IVC |
| 7 | Common iliac vein |
| 8 | Internal iliac vein |
| 9 | External iliac vein |
| 10 | Pelvic: gonadal, broad ligament veins, other |
| 11 | Common femoral vein |
| 12 | Deep femoral vein |
| 13 | Femoral vein |
| 14 | Popliteal vein |
| 15 | Crural: anterior tibial, posterior tibial, fibular veins (all paired) |
| 16 | Muscular: gastrocnemial, soleal veins, other |
| Perforating veins | |
| 17 | Thigh |
| 18 | Calf |
The descriptor (a) is added for asymptomatic patients and (s) in case of symptoms.
Diagnostic approach
The first step in evaluating a patient with venous disease is to establish his or her clinical class, which rapidly progresses from cosmetic to chronic venous insufficiency. The next is to correlate the symptoms, which place the limb being examined into one of the classes shown in Table 5.1. The patient’s clinical class will dictate the need for further evaluation. In patients with telangiectasias (class 1 or 2), the evaluation can be limited to a physical examination and evaluation of the superficial venous system with a handheld continuous-wave Doppler. Imaging of 83 limbs with clinical evidence of only telangiectatic vessels demonstrated that nearly 25% had insufficiency of the great saphenous vein (GSV) or small saphenous veins (SSV), which was not apparent on physical examination.5 Patients with symptomatic class 2 varicosities and class 4, 5, and 6 skin changes require a duplex venous reflux examination because surgical intervention is indicated.6–10 Recalcitrant cases may require more extensive imaging studies to detect venous occlusive disease. Physiologic testing can be relegated to documentation rather than to diagnosis, and phlebography should be performed only when venous reconstruction is contemplated.
Prior treatment
The physician should discuss prior treatment for venous disease. However, he or she must realize that although proper ligation, with or without stripping of the main saphenous trunks, implies that reflux through the saphenofemoral junction (SFJ) and saphenopopliteal junction (SPJ) has been prevented, this is not always the case. Some have proposed that in up to 27% of patients there is a duplication of the GSV;11–13 thus, the removal of the GSV may be followed by the development of varicosity in the remaining GSV, although this is not universally accepted.
In 20% to 40% the SSV has a variable termination14–17 that is not in the popliteal vein at or above the popliteal fossa. Therefore, the actual SPJ must be correctly diagnosed otherwise it will lead to an apparent rapid recurrence with varicose changes occurring in the remaining segment of the SSV and its tributaries. Finally, in a number of patients, a recurrence of varicose veins in the upper thigh may be the result of incomplete ligation and division of the other tributaries arising at the level of the SFJ or failure to accomplish the ligation flush with the femoral vein. In fact, in a review of 341 extremities that underwent repeat operations for varicose veins, Lofgren et al17 found that 61% had inadequate ligation. These facts make it imperative that, even in the patient with a history of ligation, division, and stripping, an examination for reflux through the SFJ and SPJ be performed.
Symptoms
It is not well known that presence and severity of symptoms has no correlation with the size or severity of varicose veins present. Symptoms usually attributable to varicose veins include feelings of heaviness, tiredness, aching, burning, throbbing, itching, and cramping in the legs (Box 5.1). These symptoms are generally worse with prolonged sitting or standing and are improved with leg elevation or walking. A premenstrual exacerbation of symptoms is also common. Patients typically find relief with the use of compression in the form of either support hose or an elastic bandage if they are compliant. Compliance can be a challenge. Weight loss or the commencement of a regular program of lower extremity exercise may also lead to a diminution in the severity of varicose vein symptoms. Clearly, these symptoms are not specific, as they may also be indicative of a variety of rheumatologic or orthopedic problems. However, their relationship to lower extremity movement and compression is usually helpful in establishing a venous origin for the symptoms. Significant symptoms suggestive of chronic venous disease should prompt further evaluation for valvular insufficiency and calf muscle pump dysfunction. If a venous etiology is suspected but all examinations are negative, repeat examination during a symptomatic period is warranted and often fruitful.
The recent development of an extremely painful area on the lower leg associated with an overlying area of erythema and warmth may be indicative of lipodermatosclerosis, which may be associated with insufficiency of underlying perforator veins or reflux from a proximal point. Examination for underlying perforator vein reflux should be performed. Lipodermatosclerosis may precede ulceration and has been shown to be improved by stiff compression and certain pharmacologic interventions.18
Rarely, patients with a history of iliofemoral thrombophlebitis who describe ‘bursting’ pain with walking may be suffering from ‘venous claudication’. In these patients, an evaluation for persistent hemodynamically significant obstruction, possibly treatable with venous bypass surgery, is appropriate.19
Complications of varicose vein disease
Complications such as ulceration and hemorrhage should be discussed with the patient because this provides additional insight into both the severity and the probable locations of abnormality within the venous system. A history of ulceration of the medial aspect of the lower leg should prompt further examination of the GSV trunk,6 whereas involvement of the lateral aspect of the lower leg suggests an abnormality in the SSV, in addition to the deep and perforating vein systems. A history of hemorrhage from telangiectasias in a particular area suggests further examination for underlying incompetent perforators and is an indication to treat all suspicious telangiectasias.20
Physical Examination
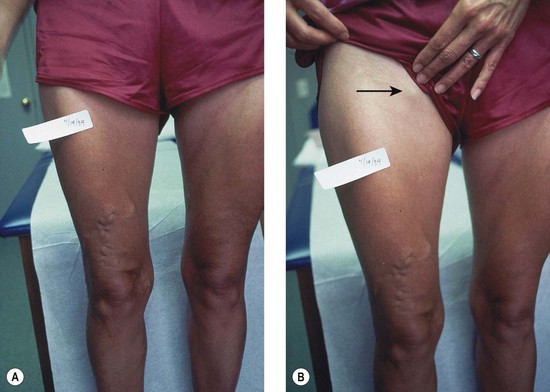
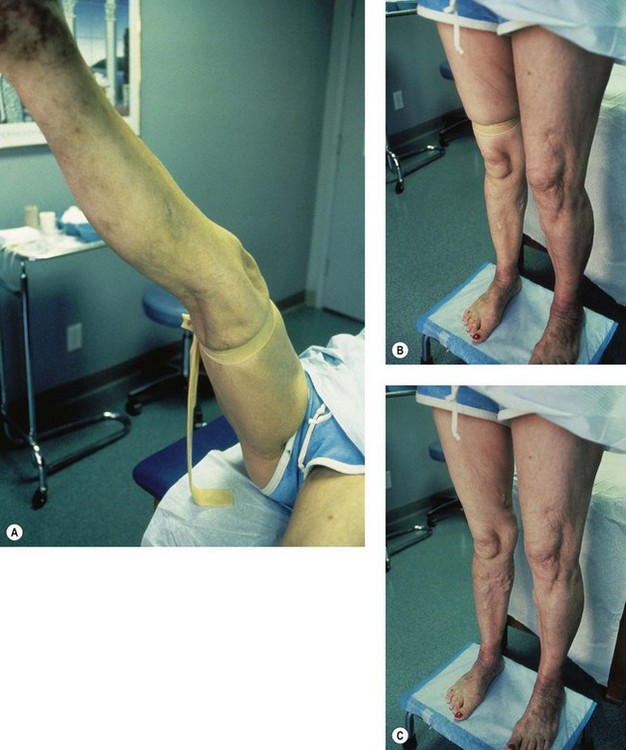
The screening physical examination consists of careful observation of the legs. Any patient with the following conditions should be examined more fully: large varicose veins; bulges in the thigh, calf, or the inguinal region representative of incompetent perforating veins (IPVs) or a saphena varix;21 signs of superficial venous hypertension, such as an accumulation of telangiectasias in the ankle region (corona phlebectatica); or any finding suggestive of venous dermatitis (pigmentation, induration, eczema). This includes patients with obvious cutaneous signs of venous disease, such as venous ulceration, atrophie blanche, or lipodermatosclerosis. An obvious but often forgotten point is the necessity of observing the entire leg and not confining the examination simply to the area that the patient feels is abnormal. The importance of this is demonstrated in Figure 5.1. This patient came for treatment of an obviously dilated anterior thigh vein, but further inspection revealed a saphena varix, with incompetence at the level of the SFJ; thus defining the first step in her treatment. Similarly, patients often seek treatment of specific clusters of telangiectasia and do not notice the underlying reticular veins that should be treated before or at the same time (see Chapter 12).
Finally, because the veins of the leg empty into the pelvic and abdominal veins, inspection of the abdomen is very important, since dilation of veins on the abdominal wall or across the pubic region suggests an old iliofemoral thrombus22 or, rarely, a developmental anomaly of the venous system.23 Dilated veins along the medial or posterior aspect of the proximal thigh or buttocks most often arise from varicosities involving the pudendal or other pelvic vessels. These can be associated with vulvar varices that may remain symptomatic after the completion of the pregnancy during which they formed. The enlarged veins in the thigh or buttocks may also be quite symptomatic and respond well to treatment.
Clinical testing
Trendelenburg test
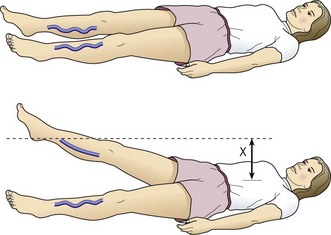
When compared with the contralateral leg, the method just described may demonstrate a degree of venous obstructive disease. Another approach is to elevate the leg while the patient is supine and to observe the height of the heel in relation to the level of the heart that is required for the prominent veins to collapse (Fig. 5.2). Unfortunately, neither procedure is sufficiently sensitive or accurate, or able to differentiate acute from chronic obstruction, which means neither of them is much assistance in current medical practice. This emphasizes the important role of duplex ultrasound in modern evaluation of the superficial venous system. One study found that pneumatic tourniquets only occluded 27% of saphenous trunks.24 Several other physical examination maneuvers, described below, can provide information on the competence of the venous valves.
Cough test
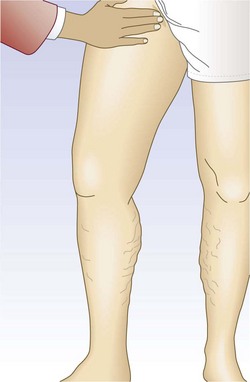
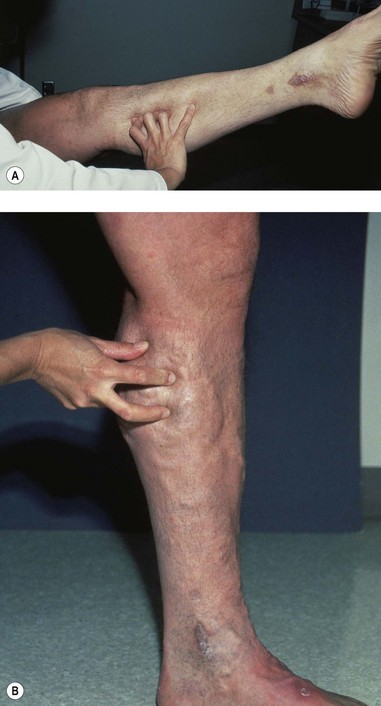
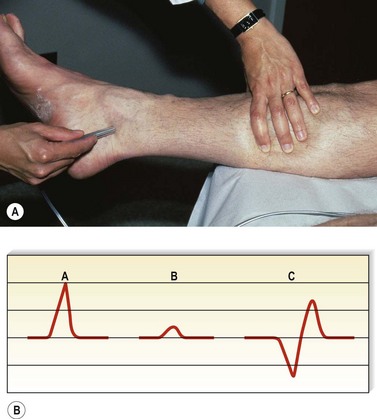
One hand is placed gently over the GSV or SFJ and the patient is asked to cough or perform a Valsalva maneuver (Fig. 5.3). Simply palpating an impulse over the vein being examined may be indicative of insufficiency of the valve at the SFJ and below to the level of the palpating hand. This test, however, is not applicable to the examination of the SSV and SPJ (see following section).14 Palpation of a thrill during this maneuver is generally more diagnostic. One study found a low sensitivity of 0.59 and low specificity of 0.67 with this test.25
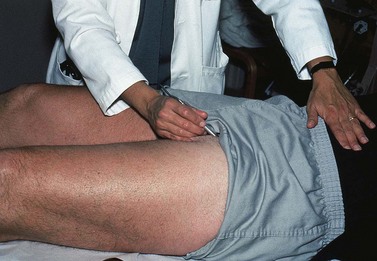
Percussion/Schwartz test
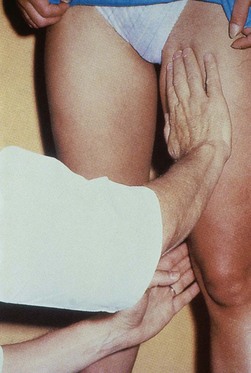
One hand is placed over the SFJ or SPJ while the other hand is used to tap very lightly on a distal segment of the GSV or SSV (Fig. 5.4). The production of an impulse in this manner implies insufficiency of the valves in the segment between the two hands. Confirmation of valvular insufficiency can be achieved by tapping proximally while palpating distally. This test can also be used to detect whether an enlarged tributary is in direct connection with the GSV or SSV by palpating over the main trunk and tapping lightly on the dilated tributary, or vice versa. The presence of a direct connection results in a palpable impulse being transmitted from the percussing to the palpating hand. As might be expected, these tests are far from infallible. In a study of 105 limbs, Chan et al26 found that these clinical examination techniques correctly identified SFJ incompetence in only 82% of limbs. False negatives were believed to be caused primarily by previous groin surgery with resultant scarring and by obesity. However, false positives were the result of variations in venous anatomy, such as a dilated tributary emptying into the common femoral vein (CFV) adjacent to the GSV or the absence of valves in an otherwise normal CFV and external iliac vein (seen in 5% to 30% of patients).27,28 Another study showed a low sensitivity of 0.59 with a high specificity of 0.92.25 A further source of error with the cough and/or percussion test is simply a misinterpretation of the muscle contraction that occurs with coughing as a reflux impulse.
Brodie-Trendelenburg test
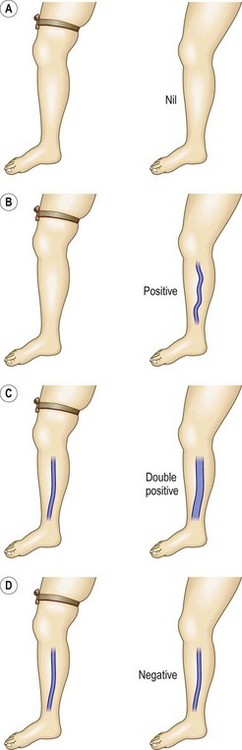
The Brodie-Trendelenburg test traditionally involves the manual obstruction of the proximal end of the GSV (or SSV) while the patient lies supine with the leg elevated, after stroking the vein in a cephalad direction to empty it of blood.22,29–32 The patient then assumes the standing position, and the leg is observed for 30 seconds (Fig. 5.5). In a ‘nil’ test there is slow filling of the veins from below, and the release of the compression does not result in rapid filling from above, indicating competence of valves in deep and perforating veins and at the SFJ (Fig. 5.6A). Rapid filling of the GSV or more distal tributaries that occurs only after release of the compression constitutes a ‘positive’ test, indicating the presence of an insufficient valve at the SFJ (Fig. 5.6B). In the ‘double-positive’ test, some distension of the veins occurs within the initial 30 seconds while the compression is maintained, as well as additional filling once the compression is released (Fig. 5.6C). This is taken as evidence of incompetent deep and perforating veins as well as reflux through the SFJ. A ‘negative’ test occurs when the veins fill within the initial 30 seconds with no increased filling after the compression is released, implying only deep and perforating valvular insufficiency (Fig. 5.6D). The reverse may not be true; that is, filling in longer than 30 seconds does not imply competence of perforating veins. In a study of 901 extremities, Sherman33 found that 95% had a nil Trendelenburg test, but surgical exploration later showed incompetent perforators in 90% of these patients. Another study showed a high sensitivity of 0.91 with a low specificity of 0.15.25
The Brodie-Trendelenburg test thus can be an important method of localizing the most proximal site of reflux in most dilated superficial veins by obstructing the GSV, SSV, or whichever vein is suspected of refluxing into a more distal vein. The physician can also place the examining finger over palpable fascial defects in the leg while the patient is supine and then release the obstructions one by one after the patient is standing. This allows the sites of insufficient perforators, or ‘points of control’ (considered so crucial in Fegan’s technique of sclerotherapy), to be defined, because the superficial veins distal to the insufficient perforator fill rapidly once the obstructing fingers are removed (see Chapter 9).34,35
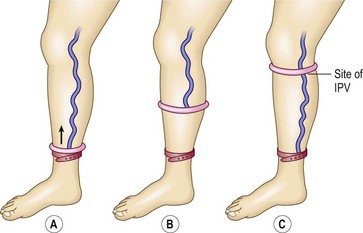
With this technique, described well in many papers,21,34–37 the practitioner first marks on the leg the sites of all dilated varicosities. The patient then assumes the supine position with the leg elevated to approximately 60 degrees to empty the veins. After at least 20 seconds, or when the distended veins are flattened, the leg is gently and rapidly palpated to detect any defects in the fascia. With experience, these can be detected easily as places that allow the entrance of the examining finger without the use of any pressure. Fascial defects can be caused by many abnormalities other than perforating veins, thus the practitioner continues the examination by compressing the individual fascial defects with his or her fingers and then having the patient stand. The fingers are then released one by one, starting with the most distal defect, and rapid filling of more distal varicosities is noted (Fig. 5.7). Those defects that cause distal filling when released are assumed to correspond to sites of IPVs. In the presence of a dilated GSV or SSV, these points of reflux first must be controlled with either digital compression or a tourniquet to evaluate the lower volume reflux through the perforators. For the evaluation to be helpful, compression of the defects must first cause sustained flattening of the varicosities when the patient initially stands. If the veins fill before any of the fingers are released, the test must be restarted and other sites compressed until the sites responsible for the reflux are located. This examination is associated with a 50% to 70% accuracy36–39 compared with findings at surgical exploration. Repeated examination at different times and improvement of edema allows the detection of increased numbers of perforators.
Bracey Variation
A clever variation of the Brodie-Trendelenburg technique was proposed by Bracey40 in 1958 (Fig. 5.8). He used a flat, 3.8-cm-wide, rubber tourniquet and two rubber rings covered with latex, with inside diameters of 7 cm and 8.2 cm. The smaller ring is used between the ankle and knee and may also be used for the thigh if the patient is thin. If not, the larger ring is used for the thigh. With the patient standing, the small ring is rolled over the foot to just above the ankle, and the rubber tourniquet is then placed below the ring to obstruct any upward flow of blood through the superficial veins. The small ring is then slowly rolled upward, emptying the superficial veins as it moves. As soon as it passes an IPV, the blood enters the superficial vein that connects with it, causing a dilation of the vein. The exit site of the perforating vein may then be marked. This reflux of blood can be accentuated by asking the patient to repetitively dorsiflex the foot. When the ring reaches the knee, the tourniquet is moved up to the knee, just below the ring. Either the smaller or larger ring is then used similarly to examine the thigh.
Perthes’ test
The Perthes’ test22,32,41 has several uses, including distinguishing between venous valvular insufficiency in the deep, perforator, and superficial systems and screening for DVT (Table 5.2). To localize the site of valvular disease, the physician places a tourniquet around the proximal thigh with the patient standing. When the patient ambulates, a decrease in the distension of varicose veins suggests a primary process without underlying deep venous disease because the calf muscle pump effectively removes blood from the leg and empties the varicose veins. Secondary varicose veins do not change caliber (if there is patency of the deep venous system) because of the inability to empty blood out of the veins as a result of impairment of the calf muscle pump. In the setting of a concurrent DVT, they may increase in size. If there is significant chronic or acute obstructive disease in the iliofemoral segment, the patient may note pain (venous claudication)42–44 as a result of the obstruction to outflow through both the deep and superficial systems. Information regarding the presence of deep venous valvular insufficiency and thrombosis is important to note in patient selection. This avoids causing catastrophic complications, such as pulmonary embolism resulting from an undiagnosed and worsened DVT or venous claudication caused by further impairment of venous return. Indeed, these two complications are serious enough to warrant the use of a much more sensitive and accurate method; therefore the Perthes’ test is now of more historical than actual clinical importance.
| Finding | Interpretation |
|---|---|
| Decreased diameter of varicose veins | Primary varicose veins |
| No change in diameter of varicose veins | Secondary varicose veins |
| Deep venous patency | Impairment of calf muscle pump |
| Increased diameter of varicose veins | Deep venous obstruction |
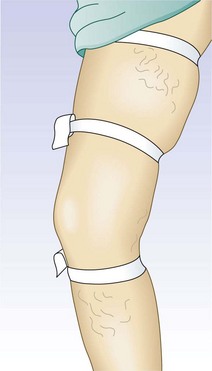
To test for perforator valvular defects, the physician may embellish the traditional Perthes’ test by placing a tourniquet around the calf just below the popliteal fossa.34 If the dilated superficial veins in the calf and ankle become less prominent as the patient ambulates, this implies that the blood is being drawn into the deep system through competent perforating veins. However, if the veins become increasingly dilated, the perforating veins must be incompetent. A more involved test, the Mahorner-Ochsner comparative tourniquet test, similarly localizes the site(s) of reflux by observing the leg while the patient walks with the tourniquet placed at various levels on the leg (upper, middle, and lower thigh) (Fig. 5.9).32
Noninvasive Diagnostic Techniques
The preceding three decades have been very fruitful and have provided a wealth of noninvasive technology that has revolutionized vascular diagnosis. A thorough description of all these techniques is certainly beyond the scope of this book, but those not presented here may be found in several excellent texts.5,13,45 Some of the new technologies have real use in the everyday performance of sclerotherapy, and the following discussion attempts to acquaint the reader with their uses and limitations.
Doppler ultrasound
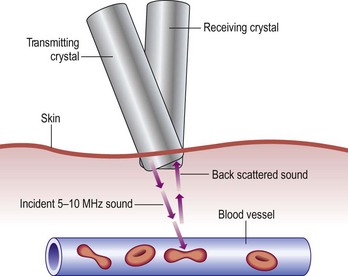
Although rapidly being replaced by duplex ultrasound, the most practical instrument for evaluating patients with venous disease is Doppler ultrasound. Its first vascular application came in 1960 when Satomura and Kaneko46 described a method of studying changes in blood flow in peripheral arteries using an ultrasonic blood rheograph. Its use in the field of venous disease was promoted by many groups, including Sumner et al,47 Strandness et al,48 Felix and Sigel,49, Sigel et al50–54 and Pourcelot et al.55 The instrument is based on the principle of the Doppler effect and consists of an emitting crystal and a receiving crystal. Sound waves are directed into the limb and reflected off the blood cells traveling through the vessel being examined (Fig. 5.10). The input picked up by the receiving crystal may be connected to a variety of audio or graphic recording systems. Dopplers come with either continuous or pulsed-wave ultrasound beams; the continuous-wave Doppler is adequate for venous examination, even though the signal represents a composite of the flow in all vessels in the path of the ultrasound beam. Thus, selective examination of one particular vessel may not always be possible. Pulsed Dopplers are used in sonar systems and in medical ultrasound imaging and are required when the intent is to focus the beam at a particular depth. Dopplers are also available in either directional or nondirectional forms. The directional type is capable of determining the direction of blood flow and depicts the direction on the tracing as either a positive (toward the probe) or negative (away from the probe) deflection (Fig. 5.11). Although the directionality greatly simplifies the interpretation of the tracing, experience with a nondirectional Doppler allows the examiner to make this determination easily, based on certain augmentation maneuvers.
The transmission frequency of the ultrasound beam may range from 2 to 10 MHz; the depth of penetration varies inversely with the frequency. Therefore, a frequency of 4 MHz produces a broad beam with deep penetration, which is especially useful for examining the deep veins in the pelvis and abdomen. A frequency of 8 MHz is much better suited for the examination of more superficial veins, including superficial segments of the deep veins of the legs, since it produces a narrower beam with relatively less penetration. Dopplers used for evaluation of the venous system generally permit detection of flow rates as low as 6 cm/second.51
Characteristics of Doppler waveform
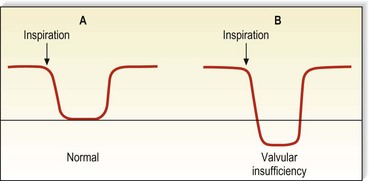
Venous Doppler signals display five characteristics (Box 5.2). In a normal patient, there should be a spontaneous signal over any vessel not otherwise vasoconstricted, and the flow should be only unidirectional. This signal diminishes in intensity with inspiration as descent of the diaphragm causes a rise in intra-abdominal pressure, thus decreasing venous outflow from the leg. It will be augmented similarly with exhalation. This waxing and waning of the intensity of the signal with the respiratory cycle is a phenomenon known as phasicity. Venous signals are continuous except for their respiratory variation and are not pulsatile, except in the setting of elevated right heart pressure such as congestive heart failure or tricuspid insufficiency56 or in the normal CFV.57 Finally, and most important to their usefulness in the evaluation of patients with varicose veins, venous signals may be augmented with certain compression maneuvers. It is the response to these maneuvers that provides information regarding the sites of valvular insufficiency and obstruction of the venous system.
By compressing the limb distal to the Doppler probe (Fig. 5.12), the examiner increases the flow through the vein; an immediate increase in the signal intensity should be heard if there is no proximal obstruction. In the presence of a hemodynamically significant DVT, the augmented response is weaker and delayed compared with the contralateral side. With the patient in the upright position, release of distal compression should be followed by silence as the valves close in response to the downward pressure of the blood being pulled by gravity. With the patient in the supine position, release of the compression should normally be followed by the return of the lower intensity spontaneous signal or by silence in the smaller veins. In the setting of valvular insufficiency at the level of the Doppler probe, a loud reflux flow signal can be heard on release of distal compression as blood is pulled in a caudal direction by gravity. To quantitate this reflux flow, the compression used may be standardized by using a pneumatic cuff inflated to a standard pressure (e.g. 80–120 mmHg), and the amplitude and duration of reflux may be read from the tracing obtained. To be considered true reflux and not merely delayed valve closure, the duration of reflux must be at least 0.5 seconds.8,58 Although many now believe that over 1 second is the appropriate duration above which to consider it abnormal.
The other method of augmentation is proximal compression and release (Fig. 5.13). Proximal compression produces a transient obstruction to outflow and thus causes an accumulation of blood distally, with an associated interruption of the Doppler signal. On its release, the large bolus of blood flowing past the Doppler probe creates a loud signal. This has also been found to be the more sensitive maneuver in diagnosing DVT, even that limited to calf veins, with a diminished or delayed signal indicative of a significant thrombosis.59,60 Valvular insufficiency is discovered easily, because proximal compression yields a loud reflux flow instead of silence.
In early descriptions of the use of Doppler ultrasound for detection of venous disease, Sigel et al51 named the various sounds ‘S’ for spontaneous and ‘A’ for augmented. They further specified ‘A’ sounds as distal (if the compression was distal to the probe) or proximal (if the compression was proximal to the probe), and positive if the ‘A’ sound was heard directly with compression or negative if heard on release of the compression. This notation thus makes it possible for four ‘A’ sounds to be generated at each site being examined. Table 5.3 summarizes these sounds and their significance. This schema provides a useful method of categorizing these sounds; however, the ‘S’ and ‘A’ nomenclature has not found generalized acceptance. Instead, sounds are referred to as manifesting flux or reflux, antegrade or retrograde flow, patency or incompetence, etc.
Table 5.3 Interpretation of ‘A’ sounds
| Type of ‘A’ Sound | Condition If Present | Condition If Absent |
|---|---|---|
| Distal positive | Normal | Venous obstruction |
| Distal negative | Valvular insufficiency | Normal |
| Proximal positive | Valvular insufficiency | Normal |
| Proximal negative | Normal | Venous obstruction or marked valvular insufficiency |
Augmentation of the most proximal portion of the GSV and of the more proximal deep veins is accomplished either by compressing the abdomen or by using a variation of the proximal compression and release, and the Valsalva maneuver (Fig. 5.14). The rise in intra-abdominal pressure caused by descent of the diaphragm is accentuated by contraction of the intercostal muscles. In the normal patient, an abrupt closure of the valves results in silence. However, more than 38% of normal persons have a brief period of reflux at the commencement of the Valsalva.61 Also, with a weak effort by the patient, a slow retrograde flow may pass through the valve and produce a Doppler flow signal because sufficient force to cause valve closure has not been generated. Visualization of the valves using ultrasound demonstrates that these valves do close eventually, and that they are not actually insufficient.62 Therefore, the continuation of reflux through at least half of the period of compression, or at least 0.5 seconds (usually 1–4 seconds), is important in diagnosing pathologic valvular incompetence.8,58 A less sensitive, but perhaps more specific, response may be elicited simply with deep breathing. With valvular insufficiency, instead of hearing the cessation of flow as the patient takes a deep breath, flow is reversed and a continuous signal heard, which shows a reverse deflection on a directional Doppler tracing (Fig. 5.15). The Valsalva maneuver is sometimes hard to explain to patients, and difficult to standardize; to facilitate, several tricks have been proposed, for example blowing into a surgical latex glove.63 When using the Valsalva maneuver to produce reflux while listening over more distal veins, the examiner must realize that the path of the reflux may be either straight down the superficial vein or through the deep vein to the perforating vein and into the superficial vein (Fig. 5.16).64 Therefore, additional testing is necessary to further delineate the exact site of abnormality. This is easily accomplished by manually obstructing the superficial vein; if reflux is still heard, the retrograde flow is assumed to be traveling through the deep and perforating systems.
Doppler examination technique
Femoral Vein
With the patient supine and the hips slightly flexed and externally rotated, the physician first locates the pulsatile signal of the femoral artery in the groin. If desired, the examination can also be performed with the patient standing, which may provide a more physiologic evaluation because most symptoms occur when the patient is upright and reflux is more easily elicited. The Doppler probe is then gradually angled medially until the spontaneous, continuous sound of the femoral vein, suggestive of a windstorm, is heard. Clear phasicity with respiration should be detected easily. Patency can be further tested by manually compressing the thigh or calf and listening for a strongly augmented signal. Valvular competence may be assessed by listening first for the phasic waxing and waning of the signal that, in severe cases of insufficiency, shows a decrease in intensity of the signal followed by a reversal of flow direction as inspiration progresses, rather than the expected silence. The patient is then asked to perform a Valsalva maneuver; alternatively, the physician can press on the abdomen. These latter maneuvers should cause an abrupt closure of the valve and silence, followed by a more intense antegrade flow on release if the valves are competent. A loud reflux flow heard through the Valsalva maneuver is pathognomonic of valvular insufficiency, which may be present in 5% to 30% of normal patients and, in one study, was found in 100% of patients with bilateral GSV varicosities.65 The effort invested in the Valsalva maneuver may be standardized to ensure the proper force and reproducibility by asking the patient to blow into a tube connected to a mercury manometer until the mercury column rises to 30 mm.
Differentiation of femoral from saphenous veins
Because the SFJ is located close to the femoral artery pulsation, valvular incompetence at the junction can sometimes be mistaken for CFV insufficiency. Several techniques can be used to aid in making this important differentiation. The saphenous vein is much easier to compress than the femoral vein, so manual compression using the Doppler probe may occlude the saphenous vein and allow the physician to listen selectively to the femoral vein. A separate occlusive device, such as the physician’s other hand or a tourniquet, may be used to compress the GSV distal to the Doppler probe and thus prevent reflux through it. Any reflux still heard is assumed to be through the femoral vein. Finally, moving or angling the Doppler probe in a cephalad direction may enable the physician to direct the ultrasound beam away from the saphenous vein to a more proximal segment of the femoral vein. Still, there are a small number of patients in whom differentiation of femoral from junctional signals may be impossible to determine by use of only the continuous-wave Doppler; an imaging procedure such as duplex scanning, which uses a pulsed ultrasound beam, may be necessary in such cases.66,67
To achieve uniform testing of venous reflux between institutions, comparable methods of testing by duplex and Doppler ultrasound scanning are desirable. In one study, the Valsalva maneuver was compared with rapid cuff deflation performed in the 15-degree reverse Trendelenburg position and in patients’ standing. Duplex technology allowed estimation of duration of retrograde flow and peak velocity. The general conclusions of the study was that the Valsalva method is best performed in the reverse Trendelenburg position as opposed to standing, but the cuff technique is more effective in the standing position.68
Popliteal Vein
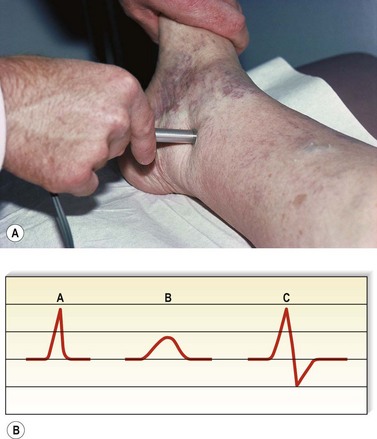
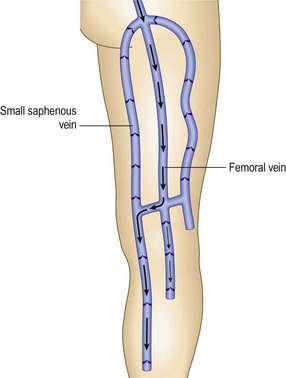
For the next site of examination, the popliteal vein, the patient may be in the supine, prone, or standing position. The most physiologic position is standing, and it is advisable to perform all presclerotherapy Doppler examinations in this position. It is important to have the knee slightly flexed, however, since full extension of the knee joint may cause a functional obstruction of the popliteal vein. Also, if the examination is performed while the patient is standing, the weight should be borne on the opposite foot (Fig. 5.17). The pulsatile arterial signal is located, generally, in the popliteal crease just lateral to the midline; the Doppler probe may be angled medially to find a softer, although spontaneous, venous signal, or it may be left over the popliteal artery. Augmentation with either calf compression or thigh compression and release, as described previously, discloses both obstruction and valvular insufficiency. The Valsalva maneuver discloses reflux only if the more proximal deep veins (CFV) are also incompetent. As with reflux heard at the femoral level, reflux at the popliteal level may actually be caused by reflux through the SPJ. Therefore, in any patient who appears to have reflux through the popliteal vein, the test should be repeated while firm manual compression is applied to the SSV. Obliteration of the reflux in this manner localizes the site of reflux to the SPJ and not to the popliteal vein itself. Another method consists of slightly compressing an uninvolved portion of the calf with one finger, which causes flow through the popliteal vein and not the SSV.64 Popliteal vein reflux can be detected in this way with a sensitivity of 100% and a specificity of 92%, with most false positives being the result of variations in the anatomy of the SSV (see Chapter 1).8,15,69 The presence of popliteal valvular insufficiency is an important finding because it is associated with diminished calf muscle pump function and may be the most important prognostic factor in the development of venous ulceration.8,70,71 This relationship is not absolute, however; one study showed that popliteal incompetence was found in only 20% of patients with ulceration and 31.2% of postphlebitic legs.61
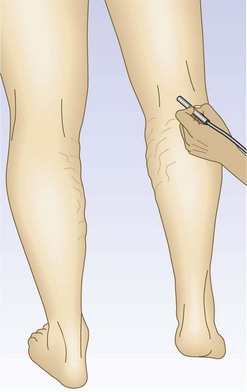
Figure 5.17 The popliteal vein is examined with the knee flexed and the weight borne on the opposite foot.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree