11 Neurophysiologic Testing for Pelvic Floor Disorders
Gynecologists frequently encounter patients with abnormal bowel, bladder, and sexual function. Normal pelvic visceral function depends on the complex interactions of intact somatic and autonomic nervous systems. Several diseases or injuries of the central and peripheral nervous systems can result in alterations in pelvic visceral function (Box 11-1). The clinical evaluation of patients with urinary incontinence, fecal incontinence, voiding dysfunction, defecatory dysfunction, or pelvic organ prolapse should include a history and physical examination that includes a clinical neurologic evaluation. When this evaluation suggests the possibility of an underlying neurologic condition, several neurophysiologic tests can be performed to assist in diagnosis. Commonly used tests to evaluate the autonomic function of the bladder and bowel include urodynamics and anal manometry. Tests that are used to directly investigate the integrity of the somatic innervation of the pelvic floor muscles and urinary and anal sphincters include electromyography (EMG), nerve conduction studies (e.g., the pudendal nerve terminal motor latency [PNTML]), and the electrophysiologic evaluation of the sacral reflexes.
BOX 11-1 NEUROLOGIC CAUSES OF BOWEL AND BLADDER DYSFUNCTION
ELECTRODIAGNOSTIC TESTING
The following section is intended to provide a fundamental overview of the neurophysiologic tests commonly used in the investigation of pelvic floor disorders. Some common indications for neurophysiologic testing of the pelvic floor are listed in Box 11-2.
BOX 11-2 INDICATIONS FOR NEUROPHYSIOLOGIC TESTING OF THE PELVIC FLOOR
Potential Indications (under investigation):
Electromyography (EMG)
The term electromyography is a general term that refers to methods of studying electrical activity of muscle. Various EMG techniques exist, each with its own indications, advantages, and limitations. Of the many EMG techniques, only a few have use for the study of pelvic floor disorders. EMG techniques study neuromuscular activity of striated muscles, using a recording electrode that is inserted into or placed on the surface of a muscle. Bioelectric potentials generated by the depolarization of the skeletal striated muscle are picked up by this electrode and then filtered and amplified. They are displayed on an oscilloscope for visual analysis and fed through a speaker system so that they can be monitored acoustically. Modern computer-based equipment allows for conversion of the signal into digital data that can then be easily stored, processed, and analyzed. Electromyographic studies used to evaluate pelvic floor disorders can be separated broadly into two categories: kinesiologic EMG (kEMG) and motor-unit EMG. Kinesiologic EMG is used to simply assess the activity or inactivity of a muscle, usually the urethral or anal sphincter; it is used in conjunction with physiologic tests, such as urodynamics and anal manometry, to assess sphincter relaxation during voiding or defecation. Kinesiologic EMG is also used for biofeedback during pelvic muscle rehabilitation for treatment of urinary or fecal incontinence. In contrast, motor-unit EMG is a diagnostic test used to assess the neuromuscular function of a muscle. It can differentiate normal muscle from denervated/reinnervated or myopathic muscle. Common techniques used for motor-unit EMG are concentric needle EMG (CnEMG) and single-fiber EMG (SfEMG). Motor-unit EMG that uses computer-assisted digital analysis to obtain a faster, more standardized and detailed assessment of neuromuscular function than traditional needle EMG is known as quantitative EMG (qEMG). The general principles, techniques, interpretation, and limitations of each EMG technique as it applies to the investigation of pelvic floor disorders and sacral neurologic disease are outlined in the next section.
Kinesiologic EMG
In the investigation of pelvic floor disorders, kEMG is used most frequently during urodynamic evaluations. Specifically, it is used to assess urethral sphincter and/or pelvic floor muscle activity during voiding. By simultaneously recording pelvic floor muscle activity using kEMG, and detrusor pressure using the urodynamic pressure catheters, detrusor/sphincter coordination can be assessed. The primary role of kEMG in this context is to detect the condition of detrusor sphincter dyssynergia in which a detrusor contraction is coupled with a simultaneous urethral sphincter contraction, resulting in abnormal voiding and frequent urinary retention. Similarly, kEMG can also be used to investigate coordinated relaxation of the levator ani muscles during defecation. This EMG technique has been used by some to diagnose anismus, a condition characterized by an inappropriate contraction of the levator ani complex, particularly the puborectalis muscle, during defecation resulting in obstructed evacuation. Another common use for kEMG is to provide visual and/or audio biofeedback of pelvic floor muscle activity during a pelvic muscle exercise program for treatment of urinary or fecal incontinence. Pelvic floor rehabilitation using biofeedback has long been advocated as more effective than teaching pelvic muscle exercises with verbal instructions alone, although recent studies by Burgio et al. (2002) have called this into question.
As a diagnostic test, except for the evaluation of detrusor-sphincter coordination, the relevance of kEMG is unclear, and no standardized method for performing or interpreting kEMG has been accepted. In the artificial setting of the urodynamic laboratory, many neurologically intact women will contract their urethral sphincter during voiding because of embarrassment and/or the discomfort of the urethral catheters and electrodes. Therefore, it is important to ask the patient whether she is trying to stop voiding voluntarily so that detrusor sphincter dyssynergia is not diagnosed in error. In a study of 550 consecutive patients, Blaivas et al. (1981) found that dyssynergia was found only in patients with well-defined lesions of the suprasacral spinal cord. Therefore, the diagnosis of detrusor sphincter dyssynergia made in the absence of other neurologic deficits consistent with this level of injury should be suspect. Inappropriate urethral contraction during voiding can also be a learned behavior resulting in dysfunctional voiding.
Concentric Needle EMG
ANAL SPHINCTER
Both the subcutaneous and deep portions of the external anal sphincter (EAS) are accessible for CnEMG evaluation. To study the subcutaneous portion of the EAS, the needle electrode is inserted 1 cm outside the mucocutaneous junction of the anal orifice, to a depth of 3 to 6 mm beneath the skin. The deep portion of the EAS is assessed by inserting the needle at the mucocutaneous junction of the anus, at an angle of about 30 degrees to the anal canal axis, for a depth of 1 to 3 cm. Typically, the subcutaneous and/or deep portion of the EAS are sampled in at least four quadrants, roughly divided into the upper and lower, left and right portions of the sphincter. Ideally, 20 or more MUAPs should be sampled during the evaluation (Fig. 11-1).
LEVATOR ANI
The iliococcygeus and pubococcygeus portions of the levator ani muscle complex are most easily investigated using a transvaginal approach. The muscles are localized by first inserting two fingers into the vagina and asking the patient to contract. One side of the levator complex is isolated, and the electrode is inserted using the opposite hand in at least two sites on the muscle. This is then repeated on the opposite side. Although there is no standardized location for needle insertion into the levator ani muscles, one technique that has been proposed uses the ischial spine as a fixed reference point and samples two sites relative to this easily identifiable landmark (Weidner et al., 2000). The first site is located 2 cm caudal and medial from the ischial spine. The second site is 2 cm farther medial. The puborectalis muscle can be accessed from a perineal approach. A 75-mm electrode is required and inserted approximately 1 cm posterior to the anus in the midline for a depth of approximately 3 cm.
INTERPRETATION
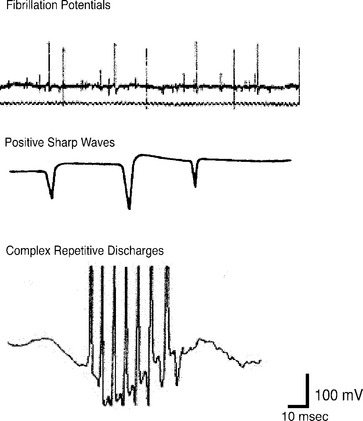
CnEMG is a valuable tool for evaluating lower motor neuron disease and muscle disease. Lesions involving the upper motor neurons in isolation have a normal EMG evaluation. The initial assessment in any CnEMG examination is an evaluation for insertional activity. Insertional activity is the electrical activity that occurs when the needle electrode is first introduced into a muscle or is moved, is the result of mechanical stimulation or injury of the muscle fibers, and usually stops within about 2 seconds of movement. The presence of insertional activity confirms that the electrode has been placed within the muscle. Absence of insertional activity with an appropriately placed needle electrode usually means a complete atrophy of the examined muscle. Once the needle has been properly placed, the patient is asked to completely relax the muscle being examined, and the presence or absence of spontaneous activity is assessed. Denervated muscle fibers may produce rhythmic spontaneous electric potentials, such as fibrillation waves or positive sharp waves. The presence of this spontaneous activity in a resting muscle is a sign of denervation. In the urethral sphincter, the anal sphincter, and the levator ani muscles, which all tonically contract even in the resting state, the only normal spontaneous activity is normal MUAPs. In limb muscles and the bulbocavernosus muscle, which do not tonically contract, there should be a complete absence of spontaneous activity at rest. The pelvic floor muscles achieve complete electrical silence during voiding and defecation. Spontaneous activity that is found in pathologic conditions includes fibrillation potentials, positive sharp waves, complex repetitive discharges, fasciculations, myotonia, myokymia, and neuromyotonia (Fig. 11-2). Fibrillation potentials are biphasic muscle fiber potentials that occur spontaneously and typically have an amplitude of between 20 to 300 μV and a duration of less than 5 msec. They usually fire rhythmically at a rate of 2 to 20 Hz. Fibrillation potentials develop 2 to 30 days after an injury and can occur with a motor nerve lesion as well as with primary muscle diseases, such as acute muscle injury, muscular dystrophies, and inflammatory myopathies. Positive sharp waves are biphasic waves with an amplitude of 20 to 500 μV and a 1- to 5-msec duration. They are more common than fibrillations and have a similar clinical significance. Complex repetitive discharges (CRDs) are spontaneous high-frequency discharges with a regular firing pattern beginning and ending abruptly that can be bizarre in appearance. They can be found after chronic partial denervation, muscular dystrophy, inflammatory myopathies, and in some metabolic disorders. The striated urethral sphincter appears to be particularly likely to develop CRDs, which have been found in association with urinary retention in young women (Fowler’s syndrome) and even in some neurologically normal women.
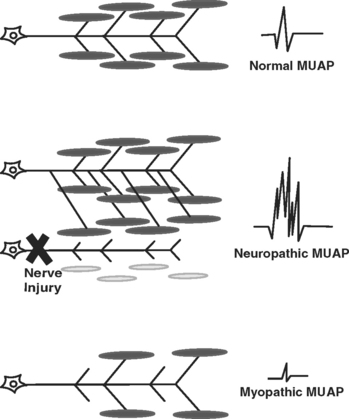
The motor unit is the smallest functional unit of the motor system and consists of a motor neuron, its axon, and all of the muscle fibers innervated by the axon. The motor unit in a normal human limb muscle consists of several dozen muscle fibers lying within an area of 5 to 10 mm diameter. The MUAP is a compound potential representing the sum of the individual action potentials generated in the few muscle fibers of the motor unit that are within the pickup range of the recording electrode. After a determination about spontaneous activity is made, the patient is asked to slightly contract the muscle being examined, and attention is turned to MUAP analysis. The shape, amplitude, duration, number of phases, and stability of each MUAP should be assessed. Most EMG units have a trigger-and-delay mechanism that allows MUAPs to be “frozen” for more careful analysis. Ideally, 20 or more MUAPs should be sampled for each muscle. This is often difficult for striated urethral sphincter, where analysis of 10 MUAPs is often all that can be achieved due to the small size of the muscle. Muscles that have been denervated and reinnervated, and muscles that are myopathic, have distinct MUAP characteristics from those of normal muscle (Fig. 11-3). The morphology of a MUAP reflects, among other things, the number and local concentration of muscle fibers comprising a motor unit. After a neuronal injury, the muscle fibers innervated by that neuron begin to atrophy. Adjacent nerve fibers attempt to reinnervate these denervated muscle fibers, resulting in a neuron that now supplies a greater number of muscle fibers. This creates a MUAP with larger amplitude, longer duration, and a greater number of phases and turns. Because these changes depend upon reinnervation by an adjacent motor neuron, large complex polyphasic MUAPs are not typically seen until 3 to 6 months after a nerve injury. In the setting of an acute nerve injury, reinnervation has not had time to occur and MUAP morphology is normal. Myopathic injury results in a loss of muscle fibers and therefore a motor unit with fewer muscle fibers. This results in a MUAP with shorter duration and smaller amplitude than normal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree