This article provides an update of the current state of motor and sensory nerve transfers for the functional reconstruction of proximal and distal nerve lesions of the upper extremity. General principles, indications, surgical options, and functional outcomes are summarized for conventional transfers used in brachial plexus reconstruction, more recently described distal nerve transfers for isolated nerve injuries in the extremity, and sensory nerve transfers performed both proximally and distally.
Key points
- •
Nerve transfers provide faster muscle reinnervation in high nerve injuries.
- •
Nerve transfers are used for both motor and sensory reconstruction.
- •
Nerve transfers are available for brachial plexus and peripheral nerve lesions.
- •
Familiarity and understanding of peripheral nerve anatomy is essential.
- •
Tension-free nerve coaptation ensures successful regeneration.
- •
Principles of donor selection are similar to those of tendon transfers.
- •
Postoperative therapy and motor re-education is required for a successful outcome.
Introduction
Nerve transfer converts a high nerve injury to a lower-level lesion that is closer to the target muscles with a greater likelihood of timely and successful muscle reinnervation. Other advantages include dissection in uninjured and unscarred tissue planes, and the need for nerve grafts is rare. Functional muscle reinnervation requires regenerating motor axons to reach their target muscles within approximately 1 year following injury. Accordingly, the outcomes after proximal nerve repair or reconstruction with grafts are frequently poor because of the irreversible loss of the target motor endplates by degeneration and fibrosis. After 1 year, irreversible architectural muscle changes begin to appear, and by 2 years muscle-fiber fragmentation and disintegration is complete, with eventual replacement by fat cells. Therefore, selection of donor motor nerves as close as possible to the target muscle(s) will help ensure muscle reinnervation before the onset of irreversible changes.
Unlike muscle or tendon transfers, because there are no tendon repairs and the muscle is left undisturbed in its anatomic bed, adhesive scar formation that restricts muscle and tendon gliding is minimal and original muscle biomechanics, vector, and tension remain unaltered. Experimental literature has shown that a tendon repair alters the recovery of muscle function to a greater extent than either a vascular or neural repair.
Denervation affects both nerve and muscle. Distal Schwann cells (SCs) are critical in promoting and directing regenerating axons through essential basement membrane proteins, and adhesion and neurotropic molecules. With prolonged denervation, SCs are less able to provide such support and may undergo apoptosis. Furthermore, the axonal transport rate decreases in an exponential fashion, resulting in the diminished responsiveness of the surviving neuron to trophic support, and the number of regenerating motor neurons may be reduced to a fraction of the original number.
Principles of nerve transfer
Nerve transfers share many common principles with tendon transfers, but there some unique considerations. The most important factors are the quality of the donor motor nerve, which correlates with the number of regenerating motor axons it will provide, and the proximity of the donor nerve to the target motor endplates to minimize the time for reinnervation. Donor nerve branches should also be redundant or expendable to minimize donor morbidity. Ideally the donor nerve function should be synergistic to the target muscle function to facilitate postoperative motor re-education and rehabilitation. Nonsynergistic or even antagonistic donor functions can be used if necessary, but postoperative therapy will be more difficult, activation of target muscle function will be less intuitive, and the functional outcome may not be as satisfactory.
Principles of nerve transfer
Nerve transfers share many common principles with tendon transfers, but there some unique considerations. The most important factors are the quality of the donor motor nerve, which correlates with the number of regenerating motor axons it will provide, and the proximity of the donor nerve to the target motor endplates to minimize the time for reinnervation. Donor nerve branches should also be redundant or expendable to minimize donor morbidity. Ideally the donor nerve function should be synergistic to the target muscle function to facilitate postoperative motor re-education and rehabilitation. Nonsynergistic or even antagonistic donor functions can be used if necessary, but postoperative therapy will be more difficult, activation of target muscle function will be less intuitive, and the functional outcome may not be as satisfactory.
Indications
Indications for nerve transfer may include an inadequate or unavailable proximal nerve stump for reconstruction, unacceptable time for regeneration by other treatment methods, prohibitive difficulty of surgery in the zone of injury, and/or an undefined level of nerve injury or lesion. Because a nerve transfer decreases the time for target muscle reinnervation, it can still be considered for later time periods of up to 8 to 10 months after injury. There has also been more recent application of nerve transfers to the management of obstetric brachial plexus palsies. Sensation can also be restored with nerve transfers. However, as experience with distal nerve transfers has grown, clinicians have become more comfortable and partial to its use in patients for whom other reconstructive options are available, including nerve graft reconstruction and tendon transfers.
Preoperative planning
Electrodiagnostic studies are helpful in determining the need and timing of nerve transfers. Nerve conduction studies may show the lack of an action potential across the lesion site, and generally indicate that intervention will be needed to recover function. Electromyography is best used serially to monitor for the spontaneous return of function by demonstrating motor unit potentials (MUPs) in the target muscle, which will be seen before any clinical evidence of muscle reinnervation. The lack of MUPs after several months will indicate that no return of function is forthcoming, and surgery should proceed to reinnervate target muscles in a timely fashion.
Technique for nerve transfer
Nerve transfers generally use a direct end-to-end nerve coaptation. End-to-side transfers can be used for noncritical sensory nerve transfers if donor availability is limited, or to restore protective sensation back to the donor territory. Intraoperative confirmation of the motor deficit and the quality of the desired donor nerve branch by electrical stimulation is essential, as physical findings and electrical studies may have confounding factors. Therefore, initial use of long-acting paralytic medications and local anesthetics should be avoided until the nerves have been identified and evaluated. Similarly, tourniquet time should be avoided or limited to 30 minutes or less to minimize the compromise of nerve function by ischemia. The recipient target nerve should be evaluated first to avoid any further surgery if it is found to be recovering.
The donor and recipient nerve branches are mobilized as much as possible, and transection of the donor nerve is done as distal as possible and the recipient nerve as proximal as possible to maximize length for a direct tension-free nerve coaptation without postural compensation. Nerve grafts may be necessary for some proximal nerve transfers, such as for brachial plexus reconstruction, and will permit better regeneration than a direct transfer with excessive tension. Purely motor nerve branches or fascicles are the preferred motor donors. For fascicular transfers, the level of the internal neurolysis and fascicular dissection on the mixed nerve should be performed as close as possible to the target recipient nerve to minimize the extent of neurolysis required and the regeneration distance. Ideally the donor nerve should have normal function, but if donors are limited, an injured but recovered donor nerve or even an injured but recovering nerve with partial function can be used if good functional recovery is anticipated.
Direct nerve transfers with sufficient laxity throughout the full range of joint motion do not require immobilization, or only for a brief period for reasons of comfort. If the pectoralis major has been detached from the humerus, the shoulder is immobilized in adduction for 4 weeks. Physical therapy will help to recover passive range of motion. Target muscle reinnervation will not generally be clinically apparent for up to 6 to 8 months or more, depending on the length of regeneration required. Once target muscle contraction is noted, therapy is used for strengthening and motor re-education to stimulate cortical remapping and more intuitive activation of target muscle.
Motor Transfers
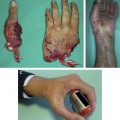
In brachial plexus reconstruction, recovery of elbow flexion and shoulder abduction is the main priority. In order of decreasing preference, donor nerves for elbow flexion include ulnar (flexor carpi ulnaris) and median (flexor carpi radialis [FCR]) nerve fascicles (double fascicular transfer to the biceps and brachialis branches of the musculocutaneous nerve) ( Fig. 1 ), medial pectoral nerve branches, thoracodorsal nerve, distal accessory nerve, and intercostal nerves. The distal accessory nerve is also the most preferred donor for the suprascapular nerve through a posterior approach for shoulder abduction and external rotation. Potential donors for the axillary nerve, also in order of decreasing preference, include a triceps branch from the posterior approach ( Fig. 2 ), medial pectoral nerves, and intercostal nerves. The thoracodorsal nerve can be considered, but is antagonistic to shoulder abduction.
Distal nerve transfers in the forearm have also been recently described. For radial nerve palsy, the FCR or palmaris longus branches of the median nerve can be transferred to the posterior interosseous nerve for finger extension, and a flexor digitorum superficialis (FDS) branch transfer to the extensor carpi radialis brevis (ECRB) branch will restore wrist extension ( Fig. 3 ). For median nerve palsy, ECRB and supinator branches of the radial nerve are transferred directly to the pronator and anterior interosseous nerve (AIN) branches of the median nerve, respectively. The brachialis branch of the musculocutaneous nerve is also a good donor for the AIN fascicle of the median nerve at the distal portion of the upper arm ( Fig. 4 ). To recover ulnar intrinsic function after a high ulnar nerve injury, the terminal AIN branch is transferred directly to the deep ulnar motor branch in the distal volar forearm ( Fig. 5 ). If the median nerve also has been injured, distal branches of the radial nerve (extensor digiti minimi, extensor carpi ulnaris, abductor pollicis longus, extensor pollicis brevis) can also be used but are more proximally located in the mid to proximal forearm, and reinnervation is slower and less consistent.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree