Fig. 9.1
Three choices for colo-anal anastomosis. From left to right: Transverse coloplasty, the Baker side-to-end anastomosis, Colonic J-pouch
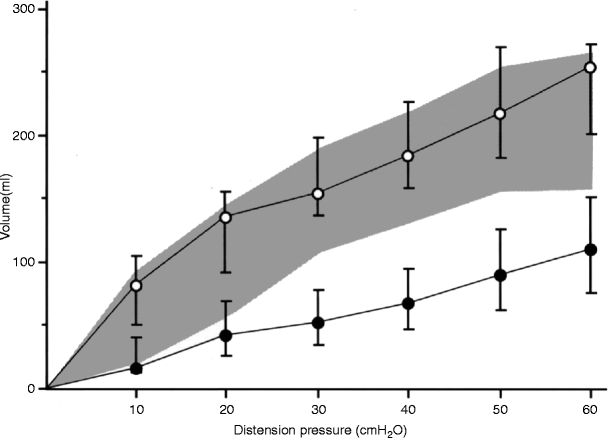
Fig. 9.2
Neorectal volumes at defined distension pressures obtained by mano-volumetry (mechanical barostat). Patients undergoing an anterior resection for cancer 1 year after restored bowel continuity. Empty circles (upper curve) represent colonic pouches fashioned with the use of a 90-mm stapler, (n = 21). Filled circles (lower curve) represent straight end-to-end anastomoses (n = 21). Values are reported as medians, with error bars representing interquartile ranges. The shaded area is the interquartile range for the healthy rectum (n = 39) (From: Hallböök [51])
Over the following two decades, numerous studies evaluated the potential benefits of the colonic pouch. In 2006, a comprehensive review comprising 1,050 patients and comparing colonic pouches and straight anastomoses was published [26]. Although this review had the potential disadvantage of publication bias because both randomized controlled trials and observational studies were included, they came to conclusions similar to those of a subsequent Cochrane analysis in 2008 [27], which limited its examination to nine randomized controlled trials assessing a total of 473 patients. In summary, both reviews showed that the pouch was superior to straight anastomosis with regard to bowel frequency, fecal incontinence, urgency, and the requirement for antidiarrheal medication up to 18 months after bowel continuity was restored. However, there were too few patients to draw firm conclusions about these advantages beyond 2 years of follow-up. There were no differences overall in postoperative complications, an issue that will be discussed in more detail later.
It should be remembered that these operations are performed for malignant disease with a cancer-specific mortality during the first 2 years, ranging from 20 to 40 % in a patient population where the median age is about 70 years. It can be argued that if a significant proportion of the life expectancy for this category of patients is to be occupied with troublesome bowel function, even a transient benefit over the first 2 years could be worthwhile. Evacuation problems with the colonic pouch have been reported in 10–25 % of patients [23, 28]. The pouches were initially made larger (with up to 12-cm-long limbs), but in more recent studies the pouch typically has been constructed using a 75-mm stapler, which provides a pouch length of about 5–6 cm (Fig. 9.1). The problem with evacuation seems to increase with an increased pouch size/limb length [29], which has been verified by two randomized trials: In one, Hida et al. [29] randomized patients with pouches between 5 and 10 cm in length and found fewer evacuation problems with the shorter pouch, without a compromise in frequency or urgency [30]. Lazorthes et al. [31] came to similar conclusions after comparing pouch lengths of 6 and 10 cm. In the series by Hallböök and Sjödahl [25], patients with a pouch constructed with a 75-mm stapler reported less evacuatory difficulty than those in whom a 90-mm stapler was used. This reduction in stapler size seemed not to have any adverse impact on frequency, urgency, or incontinence.
There are further differences in the properties of the descending and sigmoid colon that affect motility as well as have the effect on the presence of coincident diverticular disease. In addition, high ligation of the inferior mesenteric artery may render the sigmoid colon ischemic and unusable for the anastomosis. Apart from the surgical issues regarding adequate length and vascularization (which must be judged individually during the operation), there is little evidence to choose the use of either segment of the colon. In an ambitious study, Heah et al. [32] randomized patients to have a pouch constructed from either the sigmoid or the descending colon; they based the definition of either segment during the operation by marking the colon before mobilization. In that study, no differences were detected in clinical bowel function or neorectal physiology.
According to the original studies from 1986, the colonic pouch was designed for only coloanal reconstruction. Figure 9.3 shows a patient with a pouch connected too high onto the distal rectal ampulla instead of the anal canal. In this patient, the pouch had to be removed because of difficulty with evacuation.
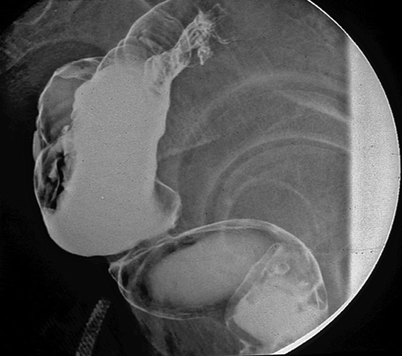

Fig. 9.3
Side view of a barium enema showing inappropriate use of a colonic pouch: the pouch is connected too high, that is, to the lower rectum instead of the anal canal. The pouch had to be removed because of severe constipation
Alternatives to the Colonic Pouch
Transverse Coloplasty
Sometimes it is not suitable, or even possible, to reconstruct the bowel continuity with a colonic pouch. This can be because of inadequate length of the remaining colon despite mobilization, bulky fatty tissue that makes folding of the bowel difficult, or a narrow pelvis with not enough space for pouch construction. The introduction of the transverse coloplasty in 1999 provided an alternate technical option for use in these patients [33]. Currently, three randomized trials have compared the J pouch with the coloplasty-pouch, resulting in similar results in bowel function during the short- and medium-term follow-up. One of these trials suggested an increased risk of anastomotic leakage in the coloplasty group [34], but these findings were not verified in a Cochrane review when the three trials were combined [27]. After the Cochrane review was written, a fourth randomized trial was published in which the J pouch was compared with coloplasty [35]. In this trial, 27 % of 364 patients, in whom it was judged intraoperatively that a J pouch was not feasible, were instead randomized between a coloplasty and straight anastomosis. At 2 years’ follow-up, the J pouch patients had fewer bowel movements, less clustering, and less incontinence than the coloplasty and straight anastomosis groups, although surgical complications in all groups were similar. The authors concluded that the J pouch offers significant advantages over coloplasty or straight anastomosis. The superiority of the J pouch showed no tendency to decline over the 2 years of follow-up.
Technically, for construction of a coloplasty, an 8- to 10-cm longitudinal incision is made starting 4–6 cm from the cut end of the colon (Fig. 9.1). The colostomy is closed transversely in a Mikulicz fashion and the colon is then anastomosed end to end to the distal rectal stump. Proponents of the coloplasty reconstruction point out that it is simpler to construct and that the evacuatory problems experienced with J pouches are less common with coloplasties, although this advantage has not been verified in available randomized trials.
The Side-to-End Anastomosis
The concept of side anastomoses in gastrointestinal surgery is well recognized. Such an anastomosis in the context of anterior resection was initially reported in 1950 by Baker [36]. The rationale at that time was to compensate for different luminal diameters.
A side-to-end coloanal anastomosis is technically simpler to fashion than a J pouch and it has the same potential advantages as transverse coloplasty in situations of inadequate colon length, obesity, and a narrow pelvic cavity. Since 1999, the side-to-end anastomosis has been compared with the J pouch in three randomized controlled trials [27]. In the trial by Machado et al. [37], the site of the side anastomosis was situated 3–4 cm from the colonic end and the anastomosis was completed by use of the transanal double stapling technique. Jiang et al. [38] used an anastomosis that was constructed from the abdomen without any transanal manipulation by a stapler to minimize the risk of anal sphincter injury, whereas Huber et al. [39] included both transabdominally stapled anastomoses and hand-sutured transanal anastomoses, with more of the latter construction in the pouch group.
Despite differences in anastomotic techniques, the randomization of patients made comparisons with a J pouch relevant with follow-up after 6 months. Huber et al. [39] found better (less) bowel frequency for J pouches, but the other bowel function variables, including evacuation, suggested similar outcomes after side-to-end compared with J pouch anastomoses; in the other two trials, patients were followed for up to 2 years [37, 38].
Neorectal Reservoirs: Why Do They Work?
Overall, it seems that except for straight anastomosis, all three alternatives for neorectal construction – the J pouch, transverse coloplasty, and side-to-end anastomosis – result in similar functional outcomes. Initially, the explanatory models were based mainly on mano-volumetric measurements, with neorectal capacity in terms of volume or compliance as part of the main focus. In this context, anorectal physiology studies of patients with an ileal pouch have had a great influence; however, the ileum is different from the colon and the fecal output from terminal ileum is also significantly different. Another way to analyze the function of the neorectum is to look at the propulsive forces inherent in the anastomotic technique, namely, to assess the pressures of the distal bowel rather than the volume.
In this regard, the issue of creating a “pressure sump” above the anastomosis was raised on the basis of studies of coloanal anastomoses using ambulatory manometry [40, 41]. More recently, neorectal motility in patients with both side-to-end and J pouch reconstructions was studied using the barostat over a period of 10 min, that is, for a longer period of time than in traditional studies of capacity. In a study by Bakx and colleagues [42], it was suggested that the functional principle of the neorectum was related more to delayed propulsive motility than to increased neorectal capacity. This may imply that the key to a well-functioning neorectum is more about breaking peristaltic activity above the anastomosis than the creation of a large reservoir. This effect can be achieved in several different ways: (1) by folding and transecting the colon; (2) by making a transverse colotomy; or (3) by simply creating a side anastomosis a few centimeters from the colonic end.
Anastomotic Leakage
Theoretically, there are a few advantages in the healing capacity of a colonic side-formed anastomosis present in both the pouch and side-to-end reconstructions. First, it has been shown that microcirculation is better preserved at the side of the colon than at the bowel end [43]. Second, with their mesenteries, the J pouch or side-to-end anastomosis can better fill the dead space in the pelvic cavity posterior to the anastomosis, potentially reducing the risk of fluid collection, which may become infected and drain through the anastomosis. Interestingly, in the original report of colorectal side-to-end anastomosis, Baker [36] found a lower leak rate with the side-to-end alternative compared with end-to-end reconstruction. Despite this, accumulated data from more than two decades of study have failed to verify any fundamental differences in postoperative complications between these techniques, including that of anastomotic leakage [26, 27].
The Use of Temporary Fecal Diversion
If the literature has failed to show any statistically significant impact on anastomotic leakage by method of reconstruction, the situation is different regarding the use of a temporary stoma. In the largest randomized trial available to date, it was clearly shown that a defunctioning loop stoma decreased the rate of symptomatic anastomotic leakage after low anterior resection when a mixture of straight, side-to-end, and J pouch anastomoses were compared [44]. A follow-up study [53] showed that the use of a temporary stoma does not influence subsequent bowel function.
Management of Anastomotic Leakage and Patency of Neorectal Reconstructions
The management of anastomotic leakage depends on the severity of the situation. A spectrum of clinical presentations occurs, ranging from the sudden, life-threatening, total dehiscence of bowel ends to a gradually developing pelvic abscess that discharges infected material through the bowel at or near the anastomosis. The presence of a diverting stoma plays an important role not only in the mitigation of these consequences, but also in decreasing the overall risk of anastomotic leakage, as mentioned earlier.
Although anastomotic leakage is the most important complication in sphincter-saving rectal resections, surprisingly little has been written concerning its management and the subsequent risks of requiring a permanent stoma. Table 9.1 summarizes different management measures for anastomotic leaks in patients with a J pouch from three different patient series [45–47]. Recently, a new technique to treat presacral abscess cavities in patients with ileoanal anastomoses has been described using transanal vacuum-assisted drainage [48]. In another series of patients with anterior resection and leakage who were treated with this technique, 22 of 29 patients were able to have their stomas eventually closed. This seems to be a promising method to help obliteration of the cavity and to avoid the formation of chronic pelvic sinuses.
Table 9.1
Management of anastomotic leakage in patients with a colonic J pouch anastomosis
Machado et al. [45] | Maggiori et al. [46] | Kruschewski et al. [47] | |
|---|---|---|---|
Total no. of patients | 161 | 200 | 128 |
Anastomotic leaks, n (%) | 17 (11)a | 41 (20)b | 15 (12)a |
Proportion of a temporary stoma at the initial operation | – | 95 % | 100 % |
No treatment or antibiotics only | 1 | 20 | – |
Percutaneous or transanal drainage | 5 | 10 | 3
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|





