Factor
Value
N
Patient sex
Male
42
Female
20
Age at initial diagnosis
(years)
Median = 74 (range 47–88)
Immunocompromised at initial diagnosis
Yes
14
No
48
Types of immunosuppression
Long-term steroids
8
Transplantation
6
Primary site of MCC
Head and neck
32
Upper limb
8
Lower limb
9
Trunk
3
Buttocks
1
Not known
9
Primary macroscopic size
(mm)
n = 53; median = 15 (range 5–60 mm)
Stage
I
38
II
4
III
20
Lymphadenectomy
Yes
17
No
45
Final surgical treatment for primary
Incisional biopsy
3
Excisional biopsy
21
WLE
29
Not applicable
9
Adjuvant RT to the primary site
Yes
43
No
10
Not applicable
9
RT to the regional node site
Yes
43
No
19
Local recurrence
Yes
9
No
53a
Regional recurrence
Yes
16
No
46
Distant recurrence
Yes
16
No
41
Not known
5
Status at last follow-up
Alive
22
Died of disease
20
Died of other/unknown
20
12.3.2 Recurrences
Nine patients (14 %) experienced a local recurrence of their MCC. Sixteen (26 %) developed a regional recurrence; all locoregional recurrences were observed in stage I or II patients. No stage III patients had regional relapses observed with a median follow-up of 44 months (range 7–115 months). Distant recurrence status was known for 57 patients and, of these, 16 (28 %) recurred in a distant site (11 stage I/II; 5 stage III).
12.3.3 Impact of RT to the Primary Site
Local recurrence-free survival was assessed. For the subset of 42 stage I and II patients, those that had RT to their primary site (n = 32) had a 2-year local recurrence-free survival of 89 % compared with 36 % for patients (n = 10) not receiving RT (Fig. 12.1, p < 0.001).
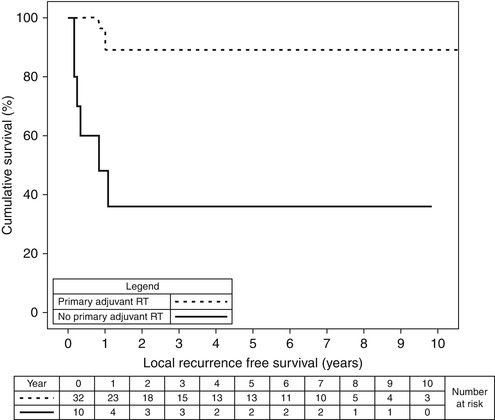

Fig. 12.1
Local recurrence-free survival of 42 stage I and II patients
Disease-free survival for RT to primary site was assessed. Disease-free survival (DFS) was significantly improved for patients having RT to their primary site. This result was observed in the overall cohort (Fig. 12.3, p = 0.009) and also the subset of patients having stage I and II disease (p = 0.048). For the overall cohort, the cumulative 2-year DFS was 54 % for the RT group compared with 25 % for the no-RT group (Fig. 12.2).
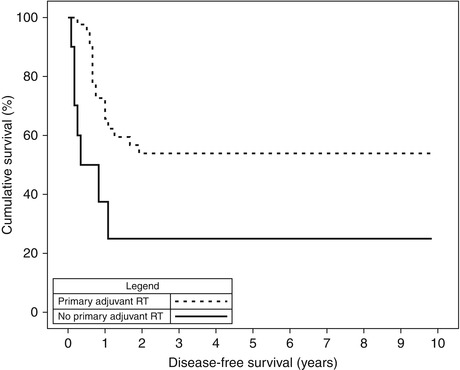

Fig. 12.2
Disease-free survival for the overall cohort of patients having adjuvant RT to their primary site (p = 0.009)









