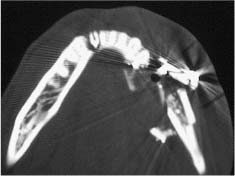
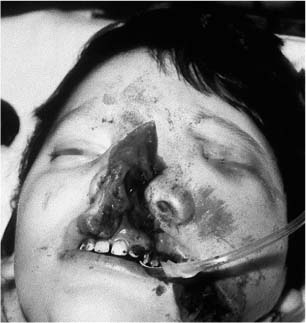
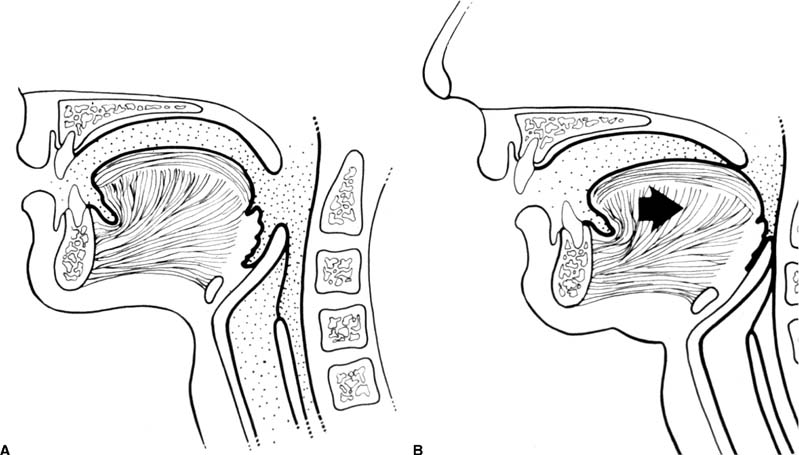
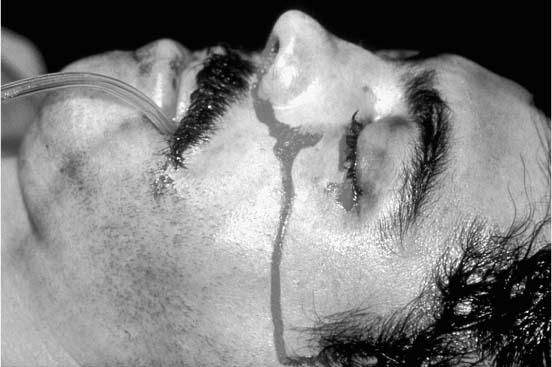
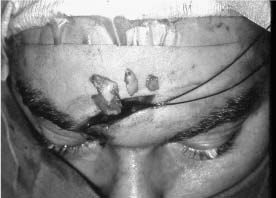
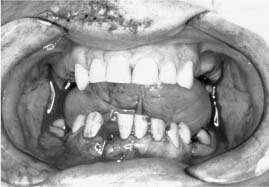
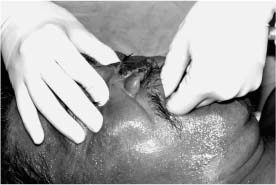
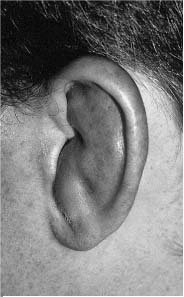
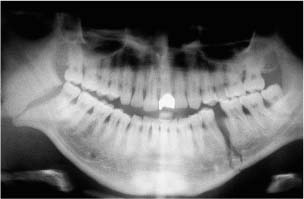
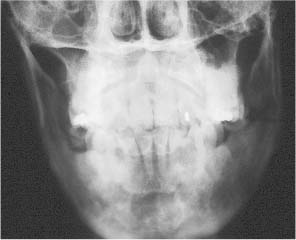
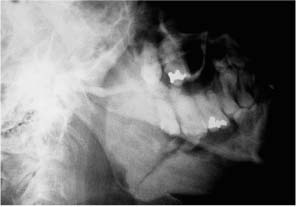
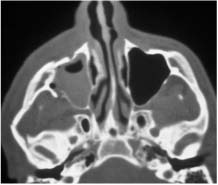
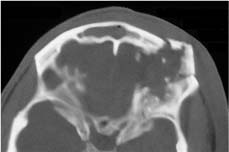
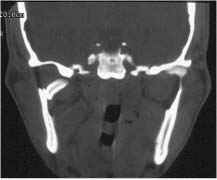
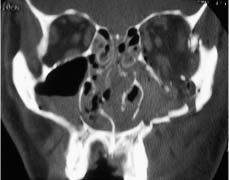
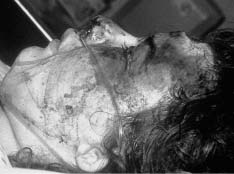
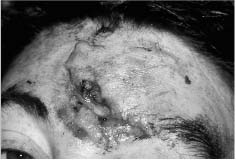
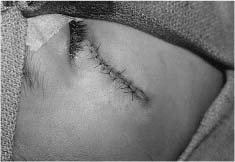
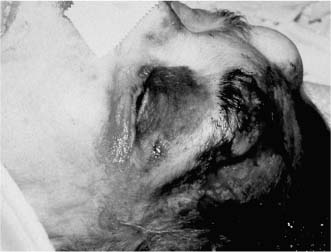
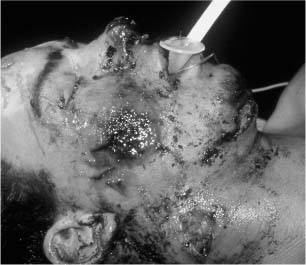
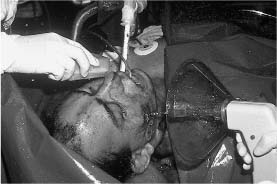
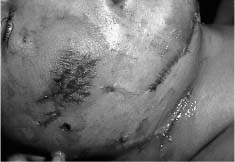
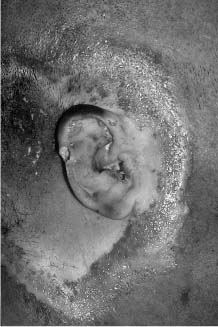
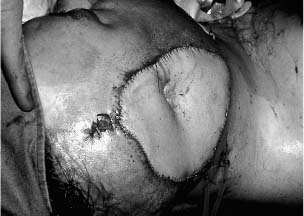
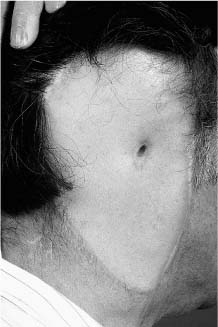
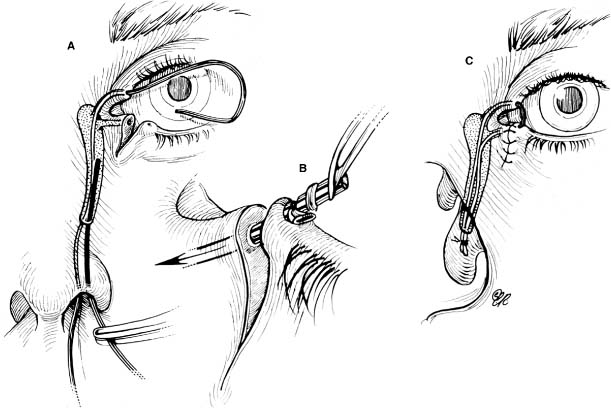
Chapter 4 To properly treat patients with maxillofacial traumatic injuries, a systematic approach should be taken toward both evaluation and treatment. This chapter reviews the basics of evaluation of the patient with maxillofacial trauma, including a reminder of the necessity for the maxillofacial traumatologist to properly understand key resuscitation requirements. Although it is beyond the scope of this chapter to completely review maxillofacial anatomy, pertinent aspects of facial soft tissue and skeletal anatomy are reviewed. In general, it is appropriate to assume that the total care of the patient with trauma is to be managed by the trauma team in an emergency department setting. However, occasionally those managing the severely injured patient with maxillofacial injuries may not have the background and training necessary to fully evaluate the patient. Accordingly, the maxillofacial traumatologist must be prepared to evaluate all systems in such a severely traumatized patient. Obtaining a through history of the traumatic event is essential, as it will assist in directing the evaluation appropriately. For example, a patient involved in a motor vehicular accident is likely to have multisystem traumatic injuries, whereas the patient who is a victim of simple assault or a direct blow during an athletic activity may require only an examination of the injured area. The examiner should be capable of providing a basic evaluation of the chest, abdomen, and extremities. When the maxillofacial traumatologist is called to evaluate a patient with maxillofacial trauma, the consulting physician should provide information regarding the stability of the patient. In the unstable patient, the “ABCs” (airway, breathing, and circulation) are strictly adhered to. Airway management is of critical importance, particularly in these patients. Although in some centers patients with significant maxillofacial trauma are routinely intubated, it is not always necessary. A cooperative patient who is breathing comfortably despite evidence of maxillofacial injury may not require urgent intubation unless later evaluation gives rise to suspicion of injuries that may lead to pending airway crisis. Conversely, in a patient who has evidence of significant maxillofacial trauma, as evidenced by significant facial swelling and obvious facial fractures, particularly in combination with evidence of chest injuries or neurologic injury, elective airway stabilization by oral tracheal intubation should be performed. In such patients there is an argument for early intubation prior to the onset of edema either surrounding or in the airway, which might make urgent intubation more difficult. Although isolated maxillofacial injuries requiring early intubation are rare, the following are examples in which an airway should be established: Bilateral parasymphyseal fractures that, because of the attachment of the tongue musculature to the lingual cortex, may allow the tongue base to drift posteriorly and occlude the hypopharynx (Fig. 4-1) A severe low maxillary fracture (Le Fort I level, particularly with a palatal split) avulsion injury with inferior and posterior displacement of this maxillary fragment A foreign body in the airway, such as a shattered denture A blast injury to the mandible or maxillary region with severe displacement and instability of the mandible and tongue region FIGURE 4-1 The tongue base can fall posteriorly with bilateral mandibular fractures, occluding the hypopharynx. (From Sinn D, Hill S, Watson S. Mandibular fractures. In: Foster CA, Shermen JE, eds. Surgery of Facial Bone Fractures. New York: Churchill Livingstone, 1987:171-193, with permission.) Oral intubation in the standard fashion is indicated and can safely be performed in the vast majority of patients. Primary nasotracheal intubation is rarely indicated, and when required for fracture management it can generally be performed in the operating room in a controlled environment. However, if for some reason this form of intubation is preferred, nasal endoscopic assistance is encouraged to confirm passage of the endotracheal tube beyond the nasopharynx into the oral and hypopharyngeal region. Endoscopic guidance is also preferred when there is suspicion of either a laryngeal injury or a cervical spine injury as any excessive or abrupt movements of the cervical spine could cause further damage. FIGURE 4-2 This patient exhibits signs of a skull base fracture with cerebrospinal fluid rhinorrhea and periorbital ecchymosis. Situations in which an acute airway emergency exists and an endotracheal tube cannot be passed are quite rare now that endoscopic-assisted intubation is widely used. However, in such an acute airway crisis where nasotracheal or oral tracheal intubation is not possible, options are few and transtracheal entry into the airway is necessary. In some centers transtracheal jet ventilation can temporarily ventilate patients long enough to take them to the operating room for an emergency tracheotomy. However, in most situations cricothyrotomy is the procedure of choice. This procedure, in which an incision is made between the thyroid cartilage and cricothyroid cartilage, should be avoided unless it is absolute essential as cricothyrotomy can be associated with laryngeal and subglottic injuries. When this procedure is performed it should be converted within 48 hours to a formal tracheotomy, whenever possible. A more common situation is the need for a formal tracheotomy in a trauma patient, such as a patient with a severe blast injury to the interior mandible and tongue region in which it is predicted that multiple procedures under anesthesia will be required. However, even in these cases it is unusual that a tracheotomy is required emergently, as an oral airway can generally be obtained followed by an urgent, yet standard, tracheotomy. After establishment of an airway, ventilation (breathing) becomes the priority. A variety of factors can cause difficult ventilation. Aspiration of gastric contents is always a problem and is worsened in the patient who has been intoxicated or has a full stomach. Accordingly, a nasogastric tube should be placed as early as possible to empty the stomach contents. A decreased respiratory drive can be the consequence of a neurologic injury, and a patient who is agitated and restless should be considered to have a neurologic injury until proven otherwise. Bleeding from the nose or oral cavity can also contribute to airway blockage and should be treated in a judicious fashion. Finally when the upper airway is controlled either by intubation or tracheotomy yet there is still evidence of ventilation difficulties, the status of the lungs should be promptly evaluated. A definite cause of ventilation difficulties can be a pneumothorax or hemothorax. Diagnosis of pneumothorax can often be made without x-rays (although a chest film should be obtained for confirmation), and placement of a needle into the superior aspect of the plural space can be acutely therapeutic. Otherwise, one can wait until a radiograph is obtained to confirm the presence of the hemothorax or pneumothorax, and a chest tube is placed between the fifth and sixth intercostals in the midaxillary line. CONTROVERSY The literature commonly states that patients with “severe facial trauma” should undergo tracheotomy. With modern intubation techniques using fiberoptic assistance, a tracheotomy is rarely necessary. Uncontrolled bleeding from the head and neck regions leading to circulatory problems is unusual, yet it occasionally occurs. Most commonly the source of bleeding is the nasal region secondary to nasal and maxillary fractures. Standard nasal packing techniques generally take care of this problem. Occasionally, when there is damage to larger vessels from the external carotid system, more aggressive packing using traditional methods or by placement of the urinary catheter with a 30-cc balloon, can be helpful in controlling the bleeding. When there is damage to larger vessels (the carotid arteries or jugular vein), the bleeding can only be controlled with digital pressure, and as definitive treatment is extremely difficult, it is important to consult with a vascular surgeon emergently Other causes of vigorous facial bleeding can be lacerations to branches of the external carotid system. Large amounts of blood can be lost through injuries to these vessels, so urgent management to tie off or suture ligature these vessels is necessary in the emergency department. If there is continued hemodynamic instability, sources other than the head or neck should be sought. These include intraabdominal injuries such as a ruptured spleen or liver injuries, as well as pelvic or extremity fractures, which can lead to significant blood loss. Although open cranial fractures require early intervention, less obvious injuries may contribute to ventilatory or circulatory problems. Accordingly, formal early neurologic evaluation and intervention by a neurologic surgeon should be obtained. Similarly, it should be assumed that all patients with facial trauma have a cervical spine (C-spine) injury until proven otherwise. Even patients with injuries indicative of minimal trauma, such as a laceration under the chin, may have a C-spine injury and should be placed in a C-collar. C-spine evaluation and clearance of injury take priority as soon as the patient is otherwise stabilized in terms of the airway, ventilation, and hemodynamics. After dealing with more acute stabilization issues, the secondary clinical evaluation and examination ensue with observation and inspection. Facial wounds are observed, measured, and carefully described in the patient’s chart. Photographs should be taken whenever possible. If examination can take place prior to the onset of severe edema of the facial soft tissues, more information can be gained. For example, once edema sets in, fracture displacement or depressions of skeletal regions such as the zygomatic arch and malar eminence may be obscured. Of critical importance is the determination of facial nerve function. As will be discussed later in the facial nerve trauma section, one has about 72 hours to determine whether facial function exists acutely. A grimace response can generally be elicited, even in a patient who is comatose, by infliction of a painful stimulus such as pressing the knuckles into the sternum. Lacerations in the cheek and medial canthal regions should lead to a high suspicion for not only facial nerve injuries but also parotid duct and lacrimal system injuries. If the patient is alert and cooperative, basic visual acuity and visual field status should be obtained. Another component of the inspection is the presence of bloody fluids from either the nose or ear. A blood-tinged watery fluid from either site may be suggestive of an anterior or lateral skull base fracture. Similarly, periorbital ecchymosis, postauricular bruising (Battle’s sign), or cerebrospinal fluid (CSF) rhinorrhea may be indicative of a skull base fracture (Fig. 4-2). The medial canthal regions are observed for symmetry and displacement, as is the external nose. The neck is observed for ecchymosis in swelling, which may indicate injury to the deep neck structures, including the larynx and trachea. Following thorough inspection of the entire scalp, face, and neck, palpation of the maxillofacial skeleton and neck is undertaken. The scalp is carefully examined for lacerations that may be hidden by hair. The forehead is carefully palpated for tenderness or step-offs. Lacerations over the forehead, nose, nasal eminence, and mandible are notorious for having associated underlying fractures. Accordingly, these lacerations should be sterilely probed during the evaluation to palpate for open fractures (Fig. 4-3). Following palpation of the forehead and supraorbital regions, the infraorbital and lateral orbital rims as well as the zygomatic arches are carefully palpated for step-offs and tenderness. Tenderness in the lateral face may be elicited in a zygomatic arch fracture by having the patient open his or her mouth. This pain may also be evidence of a subcondylar region fracture. Palpation then moves down the face to the maxillary and mandibular regions. The mouth is opened and examined for malocclusion (Fig. 4-4). This is followed by careful bimanual “rocking” of the maxilla to check for midface fractures (Fig. 4-5). It is important during this exam to note movement of the maxilla at either the infraorbital rim region (indicative of Le Fort II fracture) or along the buccal sulcus region (indicative of the Le Fort I fracture). Pain is also usually present in the same areas if fractures are present. Many midface fractures are impacted and, accordingly, are immobile. However, tenderness can almost always be elicited. FIGURE 4-3 Probing of a forehead laceration revealing comminuted fracture fragments. FIGURE 4-4 Inspection of the occlusion. Note the obvious laceration and fracture in the anterior mandible. FIGURE 4-5 “Rocking” the maxilla to determine a midface fracture. Note the left hand on the nasal root. It is then placed along the orbital rims. FIGURE 4-6 External ear auricular hematoma. Note the loss of external ear architecture as well as the obliteration of the external auditory canal by this subperichondrial hematoma. Examination continues with palpation and inspection of the external ear, looking for collections of blood, indicative of an auricular hematoma (Fig. 4-6). External canal inspection is performed to rule out damage to the anterior, inferior canal wall, indicative of a condylar impaction into the canal. Tympanic membrane inspection may find tears with or without bleeding, or a hemotympanum. These tympanic membrane injuries, particularly in combination with Battle’s sign, may be indicative of a temporal bone fracture of the lateral skull base. Examination of the various branches of cranial nerve V is undertaken using a cotton swab or pin to determine sensation in each of the divisions of the trigeminal nerve. The nose is palpated for fracture mobility, nasal stability, and tenderness. Any combination of these can be suggestive of not only a nasal fracture but also a nasoethmoid fracture (Fig. 4-7). A nasal exam is performed to observe for either acute septal fracturing or septal hematoma. The latter requires urgent treatment. Finally, the neck is a evaluated for tenderness and crepitus. Both of these findings may be suggestive of a laryngeal fracture, particularly when there is airway compromise. Following examination and documentation of facial injuries, appropriate radiographic evaluation takes place. As mentioned previously, if at all possible, injuries to the cervical spine should be ruled out either by traditional cervical spine x-rays or, as is the current practice, by magnetic resonance imaging (MRI). When no other fractures are suspected on physical exam other than mandibular fractures, the radiographic evaluation can be directed toward the mandible itself. A mandibular series should include not only a panographic radiograph of the mandible but also oblique and anteroposterior (AP) views (Figs. 4-8, 4-9, and 4-10). For the remainder of the craniomaxillofacial skeleton, computed tomography (CT) scans are the current standard for radiographic evaluation. Axial CT best delineates fractures of the cranium, medial and lateral orbits, and the zygomatic arch, as well as fractures of the mandibular symphysis, body, and angle, and fractures of the laryngeal skeleton (Figs. 4-11, 4-12, and 4-13). Coronal CT scans are best for identifying orbital floor and wall fractures, fractures through the anterior skull base, Le Fort level midface fractures, as well as subcondylar and ramus mandibular fractures (Figs. 4-14 and 4-15). Three-dimensional (3D) CT scans are occasionally useful but are generally not necessary for the management of acute maxillofacial trauma, although they are essential in evaluating the precise vertical location in subcondylar fractures. Finally, both axial and coronal CT can be helpful in the diagnosis of temporal bone fractures. FIGURE 4-7 Collapse of the nose on palpation. Note the loss of structural support in this nasoethmoid fracture. FIGURE 4-8 Panoramic radiograph with a left body fracture (comminuted) and a right angle fracture. FIGURE 4-9 Anteroposterior (AP) view of a mandibular radiograph, demonstrating a right angle fracture. FIGURE 4-10 Oblique mandibular radiograph, which best identifies fractures of the opposite mandibular angle. FIGURE 4-11 Computed tomography (CT) of the mandible, demonstrating comminution of the body. FIGURE 4-12 Coronal CT demonstrating the medial displacement of the right condyle. FIGURE 4-13 Axial CT of the frontal cranium demonstrating a fracture of the right frontal bone. FIGURE 4-14 Axial CT demonstrating multiple fractures of the left zygomatic arch. FIGURE 4-15 Coronal CT demonstrating a left orbitozygomatic fracture. The initial history should include the most recent tetanus prophylaxis as well as the history of rabies vaccination. It is often the case that the patient cannot remember the tetanus history, and so the tetanus vaccine (250-500 U) is given. In cases of an animal bite, rabies vaccine should also be given. Prophylactic antibiotics are often given in these patients, particularly when the dental alveolus and sinuses are involved in the fracture process, or if there is evidence of a contaminated wound. In the latter case, early aggressive cleaning and irrigation of the wound can be extremely useful and can prevent either acute or secondary infection. Soft tissue injuries to the facial region create a myriad of problems for the maxillofacial traumatologist. As the face is the most noticed part of the human body even small injuries may present in such a manner as to elicit an extremely emotional response, not only from the patient but also from the evaluating physician. Accordingly these injuries need to be treated with special attention to the emotional component. Many soft tissue injuries to the face are indeed severe, yet because of bleeding and their location on the face, they may be less severe than they initially appear. Conversely, even small wounds can create significant deformities, particularly when placed strategically over an important structure such as the lips, nose, eyelids, and external ear, or in the region of the facial nerve. Accordingly, the maxillofacial traumatologist must have a low threshold for becoming actively involved in the treatment of these types of facial wounds. This section discusses the evaluation and treatment of facial soft tissue wounds. We begin with a few basic principles, and then progress to evaluation and treatment of specific injuries to the important facial structures. In evaluating the facial wounds, one is often dismayed by the severity of the injuries. However, it is commonly the case that once the wounds are carefully cleaned, the surgeon often can return the soft tissues to their normal positions, and, using proper surgical techniques, the surgeon can often reconstruct the tissues in such a manner as to optimize the eventual healing and scarring. It is extremely important to use sound surgical principles in the early management of these wounds. Although most wounds present in a relatively early stage (i.e., within hours of the injury), on occasion patients present with wounds that are significantly delayed (24-48 hours after injury). Fortunately, the excellent blood supply in the facial region helps to prevent infection even in delayed wounds. Although this issue remains slightly controversial, the literature now supports wound closure even in such delayed states. The key remains proper evaluation and preparation of the wound. One must remember that every wound is potentially contaminated. Accordingly, preparation of the wound in terms of decontamination is very important. The methods for such wound preparation have been described in the literature and are beyond the scope of this discussion. However, it is safe to say that copious and vigorous irrigation of all wounds, with or without diluted antiseptic solutions, remains the mainstay for wound preparation. Conversely, use of full-strength antiseptic solutions should generally be avoided as they may further traumatize the already damaged tissues. Grossly contaminated wounds can be of several different types. The first can be a small wound with minimal abrasive components yet with infiltration of a significant contaminant. An example of such a wound might be a laceration caused by industrial machinery, in which oils and dirt may be impregnated into the wound. This type of wound requires sharp debridement of the gross contaminate in combination with significant irrigation for the less visible contaminate. At the other end of the spectrum would be a deep abrasion of the skin where contaminates can be spread over a large area (Fig. 4-16). Simple irrigation in this situation is generally inadequate. In these cases pressure irrigation, using an orthopedic pressurized irrigator, is often the best way to irrigate and debride these wounds. Sharp debridement may also be required for deep embedded foreign bodies. In any wound in which there is suspected contamination, it is wise to use a prophylactic broad-spectrum antibiotic (such as a cephalosporin) as early as possible in the evaluation of the wound. This is particularly the case in animal and human bites. FIGURE 4-16 Patient with severe abrasions of the upper face and forehead. Unfortunately, many facial lacerations are of the avulsion type, in which there is often an oblique or “skived” component to the wound in which the skin edge is not at 90 degrees to the skin surface. The skin edges in these wounds are partial thickness along the avulsed edge and may sometimes be devascularized (Fig. 4-17). There may be a tendency to aggressively debride these edges back to completely normal-appearing tissue. This more aggressive debridement is appropriate in wounds in which there is obvious excess of soft tissue adjacent to the wound (e.g., in an elder patient with lax skin in a cheek wound). However, as wound closure is the goal, many wounds cannot afford complete debridement back to totally normal-appearing skin (e.g., a young patient with an avulsion nasal wound). Accordingly, in such cases a scalpel is used to create a right-angled (non-beveled) wound edge to allow for optimal closure with wound eversion. FIGURE 4-17 A significant avulsion wound of the forehead. Note the probable necrotic area in the medial inferior aspect of the flap. For more detailed descriptions of wound closure techniques, the reader is directed to the suggested readings at the end of the chapter. However, several comments can be made here. First, some controversy remains regarding the treatment of human and animal bites. The literature now supports closure of animal and human bites after copious irrigation and institution of antibiotic therapy. Although a risk of infection in such wounds exists, the blood supply to the facial region appears to significantly decrease the chance of infection in such wounds relative to those in other parts of the body (e.g., fingers). Accordingly, bite wounds can generally be cleansed aggressively and closed unless they are several days old or obvious infection already exists. It is important, however, to maintain antibiotic coverage for at least 7 to 10 days after wound closure. CONTROVERSY Animal or human bite wounds to the face can and generally should be closed as with other wounds. Copious irrigation and antibiotic coverage is particularly important. Regarding wound closure techniques, closure of all facial wounds caused by traumatic injuries should be in a manner that one would use to close any wound in the face. Using basic principles, subcutaneous wound closure that bears the tension for the wound is followed by use of eversion suture techniques for skin closure. A vertical mattress suture technique appears to optimize this goal of eversion (Fig. 4-18). It should be noted that in lacerations in which there is tissue loss, either by evulsion or by elective debridement, there may be a skin thickness mismatch. It is important in such situations to properly place the subcutaneous sutures so as to level out the thickness of the skin edges such that a step-off deformity is avoided. The choice of suture material is of secondary importance. However, because of the difficulty of removing permanent sutures in uncooperative patients (such as in children), a resorbable suture, such as a fast absorbing gut, is often preferred. Similarly, the use of braided sutures in contaminated wounds might be avoided. FIGURE 4-18 Cheek laceration closed with adequate eversion techniques, using alternating vertical mattress and simple suture closure. Most facial lacerations can be evaluated and repaired in the emergency room setting. However, complex lacerations that require absolute patient immobilization and take longer than 2 to 3 hours to repair might be best treated in the formal setting of the operating room. Children with even less complex wounds may best be treated under anesthesia. However, techniques have evolved with anesthetics that allow increasingly complex wounds to be repaired in the emergency room setting. While taking the history and evaluating the patient’s wounds, the surgeon may be able to determine the emotional stability of the patient. Many patients are of adequate stability to undergo lengthy complex repairs without the addition of sedation. On the other hand, some patients, particularly children, may require light sedation to allow the surgeon to complete the evaluation and repair. There has been increasing use of sedatives such midazolam given either intravenously in an adult or transmucosally (intranasal or sublingual). This may provide adequate sedation to stabilize the patient during prolonged repairs. It should be remembered that nerve blocks can be very helpful in patients with large facial wounds. Accordingly, anesthetizing the three branches of the trigeminal nerve (supraorbital, infraorbital, and mental nerves) is advised, when appropriate. Topical anesthetics may be helpful in some patients. Some surgeons advocate the use of a paste made up of topical anesthetics (e.g., tetracaine, epinephrine, and cocaine [TAC]). Although TAC topical anesthesia has been found to be extremely helpful, there are several reports on its cardiac toxicity; accordingly, it is banned from many medical centers. Finally, injectable local anesthetics remain the mainstay of anesthesia and, even when nerve blocks are utilized, injection of local anesthetic into the wound margin is generally necessary. Standard combinations such as lidocaine (Xylocaine) 1% with 1:100,000 epinephrine and lidocaine 0.5% with 1:200,000 epinephrine are commonly used for this purpose. It is important to note that the purpose of the epinephrine is to provide vasoconstriction and, accordingly, one must wait 5 to 10 minutes to obtain maximum affect. Although it is commonly stated that one should not use epinephrine-containing local anesthetics in regions such as the ear and nose, this can safely be used unless there is an obvious tenuous blood supply to an avulsion segment. Hematomas occur when the tissue plane is sheered free from an adjacent tissue plane, creating a space for the potential accumulation of blood. This occurs with relative frequency, and it generally can be treated conservatively; examples are hematomas of the cheek, forehead, and scalp regions (Fig. 4-19). Although there is a tendency to aspirate such hematomas, it is generally not necessary, as blood will be absorbed without any additional intervention in most situations. The exception to this conservatism is when a hematoma occurs in either the nasal septum or external ear. In these cases, blood accumulates between the perichondrium and the underlying cartilage. Accordingly, pressure necrosis can occur with resultant instability and deformity of the structures. This can lead to a perforated septum and external nasal collapse in the nose and a deforming loss of contour of the external ear. The treatment of these specific hematomas is discussed in the sections on ear and nasal lacerations. FIGURE 4-19 Patient with a right forehead hematoma. FIGURE 4-20 Patient with severe abrasions of the face and nose. Abrasions are quite common. Most abrasions are superficial and heal quite adequately without significant intervention. However, if dirt or other types of foreign bodies are embedded within the abrasions, it is extremely important to provide superficial debridement to prevent traumatic tattooing. Although the use of a surgical scrub brush is commonly described as a way of removing such embedded debris, one must be extremely careful using such abrasion techniques. The over-aggressive use of a scrub brush may convert a superficial wound into a deeper partial-thickness or a full-thickness wound. As mentioned previously in the discussion on wound preparation, a high-pressure irrigator is most useful in such situations (Figs. 4-20, 4-21, 4-22, and 4-23). Residual debris should be meticulously removed by direct excision, and the residual oils or grease may be removed using solvents such as acetone. Meticulous posttreatment wound care is of critical importance. It has been clearly demonstrated in the wound-healing literature that a moist wound heals better than one that is allowed to desiccate. Accordingly, frequent cleaning of the wound followed by continuous moist coverage using an ointment or special topical dressing is recommended. Although it is commonly stated that antibiotic ointment should be used, the efficacy of the antibiotic component in these ointments has not been proven. Accordingly, ointments such as petroleum jelly or vitamin A and D are generally adequate and have less likelihood of a localized adverse tissue response than do the antibiotic-containing ointments. The use of such semiocclusive ointments allows for ease of wound cleaning, prevents crusting of the wounds, and allows for more rapid epithelialization of the wound. Similarly, they lessen the apparent pain caused by the wound, thus making the patient more comfortable. FIGURE 4-21 Pressure irrigation for cleansing and mild debridement. FIGURE 4-22 The same patient as in Fig. 4-21 after pressure irrigation cleaning. Note that the cheek wound has areas of full-thickness loss. FIGURE 4-23 The same patient as in Fig. 4-21 nine months after second-intention wound healing. As discussed earlier, avulsion wounds occur quite commonly. They present a variety of problems because of the devascularization that occurs at the wound edges. Many of the adverse healing aspects of such wounds are beyond the control of the surgeon. However, although debridement of wound edges is commonly necessary in avulsions flaps, one should still attempt to reposition the avulsion segment using sound wound closure techniques. The obliquely avulsed wound edges should be sharply yet minimally excised followed by use of wound eversion techniques on the skin. Avulsions that are based superiorly tend to heal adversely with a “pin cushion” type of deformity. This deformity of wound inversion in combination with thickening of the distal component of the flap is almost predictable. It is important to counsel the patient as to the likelihood of this deformity and the probable need for secondary scar revision once the wound has healed. The treatment of complete soft tissue avulsions presents a unique set of problems, both practically and philosophically. Evaluation of the wound relative to its depth and the involvement of deeper structures, the proximity to critical structures such as the lip and eyelids, and determining the patient’s ability to care for the wound are of utmost importance. Second-intention wound healing plays a major role in such avulsions. However, the avulsed wound must be in a location ideally situated for such second-intention healing. Examples of excellent locations include superficial avulsions of the forehead, temple, and occasionally the cheek, as well as small defects on the tip of the nose. These are the same locations in which second-intention wound healing would be used as the treatment for defects created after the excision of superficial skin cancers. Conversely, avulsed defects near the lips, ala, and eyelids may not be ideal for such delayed wound healing as contraction and subsequent distortion of these important structures may occur. Similarly, avulsions of tissue over the forehead or nasal dorsum may not heal adequately if bone and cartilage is exposed. But wounds may heal over such exposed bony structures if excellent wound care techniques are adhered to. The latter is completely dependent on the patients’ ability to comply with wound care instructions, and they must be aware that such second-intention healing over bone and cartilage may take many months to occur. CONTROVERSY Traditionally, second-intention healing has not been attempted over exposed bone. However, with meticulous moist wound care, such healing may occur. Soft tissue grafting may be used in the primary setting after the wound is cleaned. Composite grafts for structures such as the nasal ala may provide primary healing but may not result in an optimal cosmetic appearance. Accordingly, if such grafts are used in regions such as the scalp, cheek, temple, or forehead, the patient should once again be counseled that secondary revisions may be required. In the rare event that a segment of avulsed nose or external ear is brought with the patient, attempts can be made to repair the structures with the original avulsed tissue. The tissue should be meticulously cleaned, carefully debrided, and then sutured into place as a composite graft. Although these repairs are only occasionally successful, it is worth the effort, as the tissue may still be better than any other option, and it prevents the waste of a donor site that may be useful in a secondary repair. Finally, although local flap reconstructions are generally reserved for the treatment of secondary deformities, the use of such local tissue flaps may be appropriate in the primary setting when options are limited. This is particularly the case when significant deep structures are exposed and when questions exist as to the patient’s ability to care for an open wound. It is quite rare that patients lose large sections of soft tissue in routine accidents. However, blast injuries to the face, such as with shotgun wounds or other etiology such as burns, create very difficult treatment dilemmas. It is important to realize that patients with these severe injuries should be treated as multisystem trauma victims, and they often require basic resuscitation in addition to evaluation and treatment of the facial wounds. There are often airway and circulatory issues that take precedence. Intracranial and other neurologic injuries may be present. Blast injuries to the face inevitably have underlying structural abnormalities as well. Although it is beyond the scope of this book to discuss in detail the treatment of complex blast injuries, suffice it to say that to have adequate soft tissue reconstruction, the underlying facial skeleton must be reconstructed. Just as it is of critical importance in reconstruction of the external nose to have adequate underlying structural support, the same can be said of the remainder of the face. If structural support does not exist, collapse and devastating contraction of the facial soft tissues will occur. Accordingly, the current standard of care for blast injuries of the face involves careful debridement of obvious facial wounds, evaluation of soft tissue deficits (which are often less than expected), followed by reconstruction. Blast injuries were traditionally treated with the principal goal of obtaining soft tissue closure. Such injuries are currently treated acutely with limited debridement followed by further debridement after several days, if necessary. The full blast affect may not be fully shown for several days and, accordingly, repair after the first 2 to 4 days is appropriate. After the wound edges are demarcated, definitive debridement can be performed followed by immediate reconstruction. Although local tissue can often be used to close much of the defect in the primary setting, distant flaps are often required to provide adequate soft tissue closure (Figs. 4-24, 4-25, and 4-26). Once again, the goal in these patients is to provide coverage of important underlying structures, such as the bony facial skeleton and skull base, as well as to prevent contraction of nearby facial structures. Accordingly, the cosmetic result is less important than function and prevention of secondary contraction. FIGURE 4-24 This patient sustained an electric burn from a 10,000-V live power wire to the right mastoid and neck region. FIGURE 4-25 After debridement of necrotic soft tissues and lateral temporal bone (with dissection of the facial nerve) and placement of a scapular skin fascia cutaneous free flap. FIGURE 4-26 Six months postreconstruction. Patient deferred further scalp, neck, and prosthetic auricular reconstruction. Eyelid wounds commonly occur in maxillofacial trauma. There is perhaps no injury in the face that elicits greater emotion and should also compel the surgeon to obtain a detailed history as to the etiology of the wound. The injury can be from blunt trauma, such as the result of an interpersonal altercation or in a motor vehicle accident, or from a penetrating object. In either case one must suspect deeper injury to the globe or bony orbit. Regardless of the etiology, an ophthalmologic consultation is essential to rule out globe injury or injury to the adnexal structures. Of particular concern are globe puncture injuries, hyphema (hemorrhage into the anterior chamber), and corneal abrasions. As only an ophthalmologist has the equipment to properly evaluate these globe injuries, consultation is a must. Patients with globe injuries require aggressive treatment such as globe repair for puncture, eyelid mobilization for corneal abrasion, and strict bed rest for the high pressure created in the presence of a hyphema. Similarly, lacrimal canalicular injuries are difficult to detect without proper probe instrumentation. The ophthalmologist may be best suited to determine this injury. Extraocular muscles are occasionally injured in seemingly benign trauma as well. An often-missed injury in a setting of primary trauma is injury to the levator aponeurosis. This injury to the upper eyelid can occur even without the presence of an open wound. Accordingly, one must have a thorough understanding of the surgical anatomy of the eyelid. In the presence of a superficial wound of the upper eyelid, the wound should be explored to determine the continuity of the levator mechanism. If a laceration is vertical in orientation, a subsequent lid ptosis is less likely. However, wounds that are larger and more horizontal in orientation may likely create trauma to this aponeurosis. Repair involves reattachment of the aponeurosis or muscle fibers with small resorbable sutures. Regarding the lacrimal drainage system, one should have clear understanding of its anatomy from the superior and inferior canaliculi, which drain into the lacrimal sac and eventually into the lacrimal duct (located in the lacrimal bone), which drains into the inferior meatus. Any laceration in the medial canthal region is likely to cause an outflow obstruction. It should be noted that injuries to the nasolacrimal duct, which are commonly present with nasoethmoidal fractures, may not require primary repair as reduction of the fractured region may allow persistence of outflow after the edema has subsided. However, canalicular injuries should be treated immediately. Under magnification, the severed canaliculus is observed and then cannulated and dilated with punctal dilators. It is important that this initial repair be performed as early as possible, because with time the tissues become edematous and the canalicular stump is often retracted. If at all possible, a Crawford tube (silicone stint) is passed through the superior and inferior canaliculi and tied inside the nose in the inferior meatus (Fig. 4-27). This repair is often best performed by an oculoplastic surgeon, although a skilled and experienced facial plastic surgeon may also perform the procedure. Endoscopic or percutaneous dacryocystorhinostomy is reserved for secondary settings. FIGURE 4-27 Diagram depicting repair of a canaliculus laceration with silicone tube intubation of the superior canaliculus (A), the inferior canaliculus (B), and with tying of the tube in the nasal cavity (C). (From Lisman R, Spinelli H. Orbital adnexal injuries. In: Sherman JE, ed. Surgery with Facial Bone Fractures. New York: Churchill Livingstone, 1987:114, with permission.) The upper and lower eyelids can be considered to have two layers, the anterior and posterior lamellae. In both eyelids the anterior lamella consists of the skin and the underlying orbicularis oculi muscles. The posterior layers consist of a fibrous tarsal plate and the septum in both eyelid margins. The tarsal plate attaches to the orbital septum and underlying specialized soft tissues. On the lower eyelid the latter consists of the orbital septum and orbital fat as well as the capsulopalpebral fascia. In the upper eyelid, the orbital septum is most superficial, with deeper layers being the underlying orbital fat, the levator muscle and aponeurosis, and then the conjunctiva. Accordingly, as mentioned previously, a greater concern exists with upper eyelid injuries because of the significance of the deep musculature. Lacerations involving the margin and tarsus can be repaired systematically from the posterior lamella to the anterior lamella. The traditional description for closure of the lid margin is placement of sutures aligning the lash line, the meibomian glands, and the gray line in the posterior aspect of the laceration (Fig. 4-28). These are placed using a permanent suture that is cut with long residual “tags”so that it can be tied inferiorly so as not to scratch the cornea during the healing process. A slight eversion of the lid margin is optimal to prevent inversion or notching of the lid following the healing process. The tarsus is repaired with an absorbable suture, and the conjunctiva may be closed with a 6-0 rapidly absorbing suture. Some surgeons prefer not to close the conjunctiva. Finally, the orbicularis oculi muscle is reapproximated with a small absorbable suture. FIGURE 4-28 Repair of a marginal line laceration with sutures in the lash line, gray line, and meibomian (A and B). (From Lisman R, Spinelli H. Orbital adnexal injuries. In: Sherman JE, ed. Surgery with Facial Bone Fractures. New York: Churchill Livingstone, 1987:108, with permission.) If there is avulsion of tissue, this is generally not a problem in elderly patients with lax eyelids, as enough tissue can be mobilized without difficulty. However, when lid laxity is minimal, such as in younger patients, tissue must be mobilized to close the defect. A lateral canthotomy and inferior cantholysis generally allow for adequate mobilization to perform the repair. It has been stated that the latter maneuver may provide up to 4 to 5 mm of tissue release. When more than 5 mm of tissue is necessary, a semicircular flap (Tenzel) can close the defects of up to half of the lower eyelid and similar defects of the upper eyelid. In an acute setting, avulsed segments of the eyelid can be retrieved and resutured in position as a composite graft. Such free grafts occasionally heal. SPECIAL CONSIDERATION With any eyelid wound, particularly if there is tissue loss, remember to protect the cornea during the repair and to prescribe a lubricant postoperatively. As with the eyelid, lip lacerations often look worse than they are. Because of the tremendous vascularity in the muscle layer of the lip, significant bleeding may occur. Accordingly, careful wound analysis to locate the labial arteries should be performed so that a suture ligature may be placed. Because of the tremendous laxity of the lip, debridement to sharp wound edges is encouraged, particularly in the region in the vermilion-cutaneous junction. Classically, a suture is first placed at the vermilion-cutaneous junction. This is followed by closure of the majority of the intraoral mucosal layer. The orbicularis oris muscle is approximated with a 3-0 or 4-0 resorbable suture followed by completion of the closure of the dry portion of the red lip and securing of the vermilion-cutaneous junction. The skin is then closed with an everting suture technique. Closure of the muscle layers is extremely important to maintain sphincteric function of the lip. It is extremely rare that the upper or lower lip cannot be closed primarily. On such unusual occasions, flaps such as those described in the discussion in Chapter 2 on lip reconstruction should be consulted. The key to lip repair is precise alignment of the vermilion-cutaneous junction. Even a small mismatch becomes quite noticeable when the wound is completely healed (Figs. 4-29 and 4-30). FIGURE 4-29 Laceration of both the upper and lower lips with extension through the vermilion-cutaneous junction. FIGURE 4-30 The same patient as in Fig. 4-29 after repair. Note the good alignment of the vermilion-cutaneous junction of the upper lip but a 1- to 2-mm step-off of the scar in the lower lip. As with the lip, it is unusual that there is an avulsion of tissue from the nose. Accordingly although the initial wound may appear to involve a loss of soft tissue, careful cleaning and reposition of tissue will generally allow for adequate reapproximation and reformation of nasal tissues. However, it is imperative that a thorough evaluation be performed of the various structures of the nose. The nose is a composite structure with three layers consisting of the skin, the structural middle layer of cartilage in the lower half of the nose and the bony upper half of the nose, and the internal mucosal lining. Accordingly, the repair must be meticulous for each of these components. Similarly, debridement should be minimal as nasal skin tissues tend to be relatively immobile. As with nasal reconstruction after excisions of cutaneous malignancies, adequate internal lining is necessary for survival of avulsed cartilage-containing segments. A 4-0 or 5-0 absorbable suture should be used for closure of the internal mucosal laceration. 5-0 or 6-0 sutures are then used to reapproximate cartilage lacerations to the original position. In the unusual situation where a portion of the upper or lower lateral cartilages is missing, one might consider use of a small conchal or septal graft to reconstruct the defects in the primary setting. In a situation in which structural support has been freely disrupted by the wound (Figs. 4-31 and 4-32), and where the nose has been split in the midline, structural support should be reconstituted. This can generally be created with suture techniques (such as with a dome suture), although cartilage grafts may be necessary to add support (such as a columellar strut). Finally, the skin is closed using an everting technique. In a situation of severe internal mucosal injuries, silicone splints should be placed to prevent intranasal scarring with subsequent breathing problems. FIGURE 4-31 An upper lip avulsion continuing into a full-thickness laceration injury of the nose. Small total avulsions of the nose can be treated by replacement of the avulsed segment as a composite graft. Although delayed primary reconstruction with local flaps can be attempted, such reconstructions might best be performed in a secondary setting. Although a review of auricular anatomy is beyond the scope of this chapter, a complete understanding of the anatomy is essential for proper evaluation and treatment of external ear injuries. The intimate relationship of the skin and underlying complex cartilaginous framework is such that even small changes in the anatomy can create deformities of the ear. Accordingly, one must pay specific attention to every detail regarding anatomic relationships in external ear injuries. As a reminder, examination of the external auditory canal, tympanic membrane, and facial nerve function is also of extreme importance with respect to injuries of the external ear. The external ear sustains all types of injuries, including abrasions, lacerations, partial and total avulsions, and hematomas. Auricular hematomas are common as a result of direct blows to the external ear (Fig. 4-6). The hematoma is a subperichondrial collection of blood. It occurs due to tearing of the perichondrium from the underlying cartilage and must be treated with drainage, just as with septal hematomas. The most common sequela of an untreated hematoma is the formation of fibrosis between the cartilage and the perichondrium, which can eliminate the fine detail of the underlining cartilage. The end result is often described as “cauliflower” ear, which presents as an apparent soft tissue mass that obliterates the fine detail of the external ear. Prevention of such a secondary deformity is by evacuation of the hematoma within the first 12 to 24 hours after injury. This can be performed by aspiration of a liquefied hematoma or by placement of an incision with an anatomic landmark such as the scapha to allow for open drainage. Although further treatment is slightly controversial, in most settings a bolster is placed to close the space created by the hematoma. Generally a form of gauze with a through-and-through mattress suture provides adequate pressure to allow for healing of the subperichondrial space. FIGURE 4-32 Six months postoperative result in the same patient as in Fig. 4-31. Note excellent restoration of the upper lip and nose after meticulous multilayer closure including reapproximation of nasal cartilages. Also, note the poor scar in the left lower cheek region, typical of superior-base avulsion flaps. CONTROVERSY Although traditional treatment of auricular hematomas is with open drainage followed by placement of a sutured bolster, some advocate frequent aspirations with placement of nonsutured bolsters. Treatment of lacerations of the external ear is similar to treatment of those of the nose. One must remember that there are multiple layers including anterior ear skin, cartilage, and posterior external skin. Accordingly, the cartilage is apposed with small (e.g., 5-0) material followed by standard closure of the skin layers with particular attention to approximating landmarks such as the helix, scapha, antihelix, and crura. For lacerations extending to the external auditory canal, one must be concerned about scar contracture of the canal during the healing process. Accordingly, at a minimum, a wick should be placed and, in larger soft tissue injuries, a stent should be kept in place for a least 5 days after repair. SPECIAL CONSIDERATION With external canal injuries, stenting may limit canal scar contractures. As the external ear protrudes away from the body, it is susceptible to avulsion types of injuries. Remarkably, it is not uncommon to see a subtotal avulsion of a segment of external ear that, after appropriate initial wound management, appears quite viable in terms of vascular supply. If such an avulsion segment appears pink in color, implying both good arterial inflow and venous outflow, reattachment of the segment followed by placement of a soft protective external ear dressing would generally result in an adequately healed external ear (Figs. 4-33 and 4-34). On occasion, an external ear segment is attached to a precariously small pedicle and it is difficult to determine either the arterial inflow or the vascular outflow. If upon pinprick of the ear one notes a release of fresh (yet dark) blood, it may well imply that venous outflow is obstructed. It is in this unusual circumstance that medicinal leeches can be of extreme value. SPECIAL CONSIDERATION When a reattached avulsed ear segment is cyanotic yet bleeds upon pinprick, venous outflow is a likely etiology. Medicinal leeches may be of great value. FIGURE 4-33 Full-thickness laceration of the upper half of the right ear. The only attachment is the skin anterior to the root of the helix (arrow). As the skin remained pink, it was sutured back together in multiple layers, including cartilaginous closure. FIGURE 4-34 The 4-month post-repair result in the same patient as in Fig. 4-33. Small total avulsions of the external ear may occasionally survive as a composite graft. Protection of such avulsions using moist gauze dressing or a soft external ear (mastoid) dressing may be helpful to promote adequate healing of such avulsed wounds. In larger avulsions of the ear (if the segments are greater that ~2 cm2), decisions need to be made as to how best to manage these injuries. These large segments are unlikely to survive on their own, although primary reattachment is an option that has mixed results. Such replantation has an advantage in that no “bridges are burned” in terms of manipulation of adjacent valuable local tissue. More aggressive treatment includes the use of two types of buried segment techniques. In the first technique the skin is completely removed from the avulsed segment and then the cartilage is sutured to its position on the remaining ear remnant. A small pocket is then created in the skin adjacent to the avulsion posterior to be followed by draping the skin over this cartilage and suturing it to the residual ear skin. After several weeks, the avulsed cartilage remnant is elevated sharply, creating a postauricular sulcus, leaving soft tissue from the mastoid region on the posterior aspect of the originally avulsed cartilage. The cartilage will generally accept a skin graft. The problem with this technique is that it results in a significant obliteration of the fine detail of the external ear. A second technique for treatment of larger partial- or full-thickness avulsions is to attach the avulsed cartilage to its native position on the external ear remnant followed by deep dermabrasion of the skin overlying the segment. The segment is then buried deep to the postauricular mastoid skin in hopes that a vascular supply will be provided through the dermabraded skin. This technique is not commonly utilized even though successful results have been reported. CONTROVERSY Although there are advocates for postauricular pocket placement of ear avulsion segments, many feel that such techniques “burn bridges” in respect to saving normal skin for later use in secondary reconstruction. Total ear avulsion presents a significant problem. This problem can be treated in one of several ways. There are several anecdotal reports of primary attachment of these segments with eventual survival. Once again, this approach has the advantage of not traumatizing the surrounding tissues, but generally only a portion of the ear survives. Another technique that has been described with limited success is complete removal of the ear cartilage framework, followed by covering of the framework with a thin vascularized tissue such as a temporoparietal fascia flap. This is then covered by a thin skin graft. Although an ear structure can be re-created with this technique, there is a predictable loss of anatomic definition. There are also several case reports of successful microvascular replantation of near-complete or complete external ear avulsions. The key to this procedure is that microvascular venous reattachment is reestablished. Although the arteries to the ear are very small, they can commonly be identified and, in skilled hands, arterial revascularization is possible. However, venous revascularization is often quite difficult and, accordingly, if venous obstruction occurs, leeches can be used for several days until venous revascularization takes place. Finally, in cases of subtotal ear losses, standard techniques used in ear reconstruction for oncologic defects are applicable. These include the use of a costal cartilage framework, similar to that described in microtia repairs, covered with a temporoparietal fascial flap and a skin graft. This gives the possibility of an ear-like structure. Perhaps the best option in such cases is the use of a prosthetic external ear. Furthermore, the use of osseointegrated implants allows for an excellent, yet removable, attachment of the external ear prosthesis. Patient selection is extremely important because of the high maintenance and attention to hygiene required for such implant-borne external ear prostheses. The parotid duct has been described as exiting the anterior and superior border of the parotid gland, continuing over the masseter muscle, then abruptly turning medially toward the oral cavity. It then passes through the buccal fat pad, penetrates the buccinator muscle, and enters the lateral superior buccal sulcus as Stensen’s duct just opposite the maxillary second molar. The course of the duct can be thought of as approximating a line drawn from the midtragus to the corner of the mouth. Parotid duct injuries can be caused by a variety of injuries to the cheek region but are most commonly caused by penetrating injuries, such as in the case of broken windshield glass, a knife wound, or a penetrating bullet wound. Lacerations, particularly those orientated vertically in the midportion in the cheek, can also create a parotid duct injury (Fig. 4-35). It is likely that many parotid duct injuries are not diagnosed and are of no clinical consequence. The duct may reapproximate and become functional, or, in the case of an open laceration into the oral cavity, may fistulize into the oral cavity and reestablish function. However, it is certainly well known that a missed diagnosis of a duct injury may cause parotid swelling, salivary leaks, and even fistula formation and wound infection. The key is that there is a high index of suspicion to evaluate a wound for injury to the duct. Diagnosis of an injured parotid duct is difficult. However, when there is a high index of suspicion, one might inject methylene blue through a silicone catheter into the duct and this might be visualized within the wound. Another less reliable method is “milking”the parotid gland forward to detect extravasation of saliva flowing from the proximal cut end of the duct into the wound. FIGURE 4-35 A right cheek laceration with buccal nerve branch paralysis. This wound was explored after CT scan was obtained revealing a right zygomatic fracture. The fracture was repaired along with microscopic exploration and reanastomosis of the facial buccal nerve branches. The parotid duct was explored and found to be intact. Repair of the duct is classically described as placement of a small silicone catheter through Stensen’s duct intraorally, withdrawing it from the cut distal end of the duct, and then threading it into the cut proximal end of the duct (Fig. 4-36). The small microsuture (e.g., 8-0 nylon) can be then placed to secure the cut ends of the duct over the catheter. Some controversy exists as to the length of time necessary for the catheter to remain in place. Using precise microsurgical techniques with a watertight anastomosis, the catheter probably can be removed within a week. However, the traditional literature states that it should be left in place for 3 to 4 weeks to prevent later scar obliteration at the repair site. As stated earlier, repair of the duct is probably not necessary, as reflex atrophy or, conversely, development of new drainage pathways may occur. On rare occasions when a patient presents with late parotid swelling, salivary leak, or fistula formation, radiation therapy or even parotidectomy have been described as the treatment. FIGURE 4-36 Identification of a lacerated parotid duct, silicone tube intubation, and repair. (From Lisman R, Spinelli H. Orbital adnexal injuries. In: Sherman JE, ed. Surgery with Facial Bone Fractures. New York: Churchill Livingstone, 1987:271, with permission.) The sequelae of trauma to the facial nerve are well known. Not only can gross facial asymmetry and flaccidity be present with facial paralysis, but also the functional problems of corneal exposure, disturbances with speech and eating, drooling, and eyebrow ptosis with visual disturbance can be extremely disturbing. Accordingly, prompt recognition of potential nerve injury with facial trauma is essential. Any laceration of the lateral cheek should cause concern for the potential of a facial injury (Fig. 4-35). Accordingly, blunt probing of the wound to determine the depth of the injuries is very important. In the lateral cheek, any injury that appears to have penetrated deep to the parotid fascia should elicit concern. It is of critical importance to document facial nerve function as early as possible in evaluating the patient with a potential facial injury. Unfortunately, such documentation is commonly overlooked in the polytraumatized patient. It is common that an otolaryngologist or facial plastic surgeon is consulted regarding facial paralysis many days after the initial injury. Facial motion can be tested even in a patient who is comatose or has a grimace response to pain stimulus (e.g. knuckle pressure to the sternum). In the cooperative patient, sequential examination of each facial nerve region should be evaluated. This would include forehead function, eyelid closure, oral commissure evaluation when smiling to determine upper lip motion, and lower lip depression. When there is partial (or regional) paralysis of the face, one should suspect direct facial nerve branch lacerations. However, when lacerations involve the earlobe and posterior cheek region, nerve trunk injury is more likely, which results in complete facial paralysis. Immediate facial paralysis almost certainly suggests transection of the facial nerve and merits exploration with repair. Classically, it has been described that any laceration medial to a line dropped vertically from the lateral canthus involving facial paralysis does not require nerve repair. The classic thinking is that the more medial the nerve injuries are within the distal facial musculature, the more likely it is that the muscles remain at least partially innervated, thus having some potential for return of function. However, this has become more controversial and any wound lateral to the nasolabial fold associated with facial paralysis should probably be explored with an attempt to perform a neurorrhaphy. CONTROVERSY Although the literature often states that facial nerve injuries need not be repaired if medial to a vertical line dropped from the medial canthus, one should try to repair such injuries, if possible. At the time of the initial presentation of posttraumatic facial paralysis, the history as to the time and origin of the trauma is very important. As stated, immediate loss of function implies complete transection of the nerve. Conversely, documented delayed onset implies a neuropraxia, which should provide good prognosis for return of function. Unfortunately, it is not uncommon for the evaluating facial plastic surgeon to be confronted with a patient with partial or total facial paralysis in whom the onset and duration of the paralysis is unknown. In such cases electrodiagnostic tests are necessary. Such tests are useful in determining not only the integrity of the nerve but also the level of injury. Similarly, some of these tests may help in determining prognosis for recovery. It is well accepted that the facial nerve retains conductivity for about 72 hours after nerve injury. Most of the electrodiagnostic tests determine nerve injury only after this 72-hour lag period. Accordingly, one should perform the first test as soon as possible to determine baseline function. The most common tests are facial nerve stimulation, electromyography, and electroneurography. Using a Hilger nerve stimulator, the facial nerve is stimulated near the angle of the jaw with recording electrodes in the frontalis or the orbicularis muscles. The baseline is obtained on the normal side and then on the injured side. Readings on the involved side relative to the normal side greater than 3.5 mA suggest that nerve conduction is absent and that there is degeneration and possibly transection of the nerve. The length of latency may also suggest whether there is only a contusion (neuropraxia) of the nerve or actual partial or complete transection of the nerve (axonotmesis or neurotmesis). Electromyographic testing records muscle potentials regardless of external electric stimulation. With electrodes placed within the distal facial muscles, interferences are recorded. With no injury to the nerve, a normal facial muscle shows no electrical potentials. However, with denervation fibrillation potentials are present. After complete nerve transection, normal action potentials that are present with voluntary contraction of the facial nerve are replaced by fibrillation potentials suggestive of the degeneration. With partial nerve lacerations, fibrillation is also noted in the muscles at rest, whereas with voluntary contraction polyphasic potentials with some “giant forms” are present. This represents evidence of denervation with myopathy, as there is low voltage and an inability to recruit motor neurons on involuntary contractions, which is suggestive of a long-standing injury. Quite simply, if action potentials are not present yet fibrillation potentials exist, there is a likelihood of complete transection of the nerve. Conversely, if action potentials are present in a sudden facial paralysis, facial nerve regeneration prognosis is good, with the likelihood of good functional return. Electroneurography (ENoG) is a well-accepted tool for electrical measurement of the facial nerve. Maximal facial nerve stimulation is provided at the stylomastoid foramen and the amplitude of potentials are measured and recorded as a percentage of the unaffected side. The assumption is that the percentage is proportional to the number of degenerated fibers of the affected nerve. As opposed to electromyography, it has been determined that there is only about a 24-hour delay in ability to detect degeneration. It has been suggested that if 90% or greater degeneration is measured by ENoG, severe facial asymmetry and deformity result. Accordingly, if 90% or greater degeneration is measured by ENoG, a transection of the nerve is likely. Similarly, in presence of a temporal bone injury, this measurement is highly specific for an intratemporal bone facial nerve injury. The use of such electrodiagnostic tests continues to be controversial, and therefore clinical judgment remains the most important component to this process. Accordingly, any laceration in the posterior cheek associated with facial paralysis, particularly if it is oriented vertically, probably merits formal exploration. Such exploration can be performed through the laceration if it is large enough. Otherwise, the laceration can be combined with a standard parotidectomy incision (Fig. 4-37). The main trunk of the facial nerve is identified using standard anatomic landmarks. This dissection is particularly important in lateral facial lacerations. In more anterior cheek lacerations, exploration through the wound may be more appropriate, identifying the nerve branches in retrograde fashion. In the more posterior cheek lacerations, after the main trunk of the nerve is identified, an attempt is made to find the distal portion of the nerve. In this situation the use of a nerve stimulator can be extremely useful for identifying the distal main trunk of the nerve. Once the severed trunk or branches are identified neurorrhaphy is performed. If possible, the nerve ending is carefully freshened with a sharp scalpel blade and the nerve repair begun. Most surgeons prefer an epineural repair using a small nonabsorbable suture (e.g., 7-0 or 8-0 nylon). Atraumatic technique should be utilized and the nerve should be repaired with minimal or no tension. If significant tension is noted during the repair, placement of an interpositional nerve graft should be considered. If such an interpositional nerve graft is required, the optimal donor nerve is that of the great auricular nerve, harvested as it surfaces over the posterior aspect of the sternocleidomastoid muscle. The graft is interposed and similar epineural neurorrhaphy is performed. FIGURE 4-37 A deep preauricular laceration with traumatic facial paralysis. The forceps is holding the cut distal to the extratemporal facial nerve. In patients with facial nerve injuries, with or without neural repair, adjunctive measures should be considered. In particular, eye protection is extremely important. In a younger patient with facial paralysis, adequate eye closure and protection is generally present due to the excellent tone of the eyelid margin and skin. However, in older patients, laxity of these structures may lead to immediate or delayed inability for the patient to protect the eye. Accordingly, corneal protection is initially obtained by placement of specific ointments, and one may consider reversible procedures such as upper lid loading with a gold weight and lower eyelid tightening. Although these procedures are rarely necessary, they can be extremely helpful to the patient. If at all possible, one should avoid tarsorrhaphy as it necessarily blocks vision in the involved eye. Patients with soft tissue injuries of the face often need long-term follow-up, as was discussed with facial nerve injuries. Because of the blunt and macerated nature of many soft tissue injuries, suboptimal scarring certainly can occur. It is important during the early phases of wound healing to counsel the patient in terms of the length of time one should expect before there is final wound healing. In young children this can be as long as 18 months. Conversely, in an elderly patient with significant skin damage, this healing can be as short as 3 months. Secondary scar revisions should be planned accordingly. Although it has been stated that one should wait for an extended period of time prior to performing scar revision, each individual situation dictates when this is appropriate. The surgeon should wait at least until the inflammation phase has passed, but it does not mean that complete scar maturation is required prior to revision. The latter is particularly the case in the patient who has a significantly inverted scar, particularly if there is an adverse psychological impact. Another modality that should be considered in the early phase of wound healing is dermabrasion. Particularly in an irregular wound, dermabrasion at between 4 and 8 weeks postrepair can optimize wound healing and possibly preclude the need for later scar revision. CONTROVERSY Although it is often stated that scar revision should be delayed until there is total scar maturation, it can be performed sooner in some cases of severe deformity, especially if the scar creates functional problems. The treatment of craniomaxillofacial fractures throughout the 20th century has evolved from closed treatment of fractures to the use of interosseous wire fixation and the use of external devices. Although first described in the 1960s, the use of internal fixation did not increase in popularity until the late 1970s to 1980s, and it has really been only in the past 15 to 20 years that plate and screw fixation has gained universal acceptance. The evolution has been toward refining the metallurgy as well as developing different shapes, sizes, and malleability of the plates. More recently, resorbable plates and screws have gained some acceptance. It is quite likely that other forms of fixation such as rivets and tissue glues may well find a place in treatment in trauma. This section briefly reviews bone healing principles, the basic principles of facial plating, and the surgical approaches to the craniomaxillofacial skeleton. Healing of bone throughout the body depends on reduction and stabilization. Not unlike the skin, bone has an innate ability to heal regardless of the stabilization technique utilized. Accordingly, bone healing can be guided by external casts, splints, external fixation devices, or internal fixation devices. Most commonly, there is at least some motion (“micromotion”) at the fracture site. The initial inflammatory response of healing is followed by the formation of a callus, which stabilizes a fragment, then allows osteon growth across the fracture defect such that the fracture site is bridged by new bone. The basic principles of stabilization of fracture fragments allows this process of callus formation and eventual replacement by solid bone to occur. Accordingly, it behooves the surgeon to optimize the bone healing with an appropriate type of fixation for each fracture. This does not mean that internal or external devices are required for all fractures, but it does require the basic understanding of which fractures are most likely to heal most optimally with different types of techniques.
MAXILLOFACIAL AND SOFT TISSUE TRAUMA
MANAGEMENT OF FACIAL TRAUMA: GENERAL PRINCIPLES
PRIMARY EVALUATION OF THE MAXILLOFACIAL TRAUMA PATIENT
In these injuries, elective urgent intubation is indicated. In a patient with obvious airway obstruction caused by intraoral pathology (e.g., collapse of the tongue base or maxilla), emergency measures such as placing a clamp on the tongue and pulling it forward can be lifesaving Similarly, careful placement of a nasal trumpet to bypass a collapsed palate and tongue may also be extremely beneficial. However, in a patient with suspected anterior skull base injuries, care should be taken when placing nasal tubes to ensure that they proceed in a normal pathway of the airway instead of the superior direction (Fig. 4-2). Fiberoptic visualization can be very helpful in such circumstances.
SECONDARY EXAMINATION
RADIOGRAPHIC EVALUATION
GENERAL PREOPERATIVE CONSIDERATIONS
MANAGEMENT OF SOFT TISSUE INJURIES
GENERAL PRINCIPLES
WOUND CLOSURE
ANESTHESIA
TREATMENT METHODS FOR SPECIFIC WOUND TYPES
Hematoma
Abrasions
Avulsions
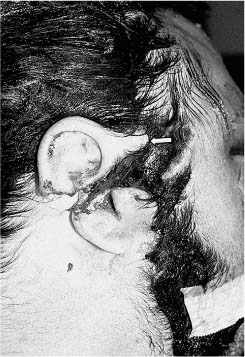
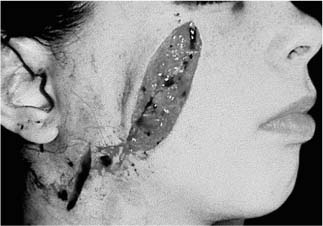
Massive Soft Tissue Losses
WOUNDS OF SPECIFIC ANATOMIC STRUCTURES
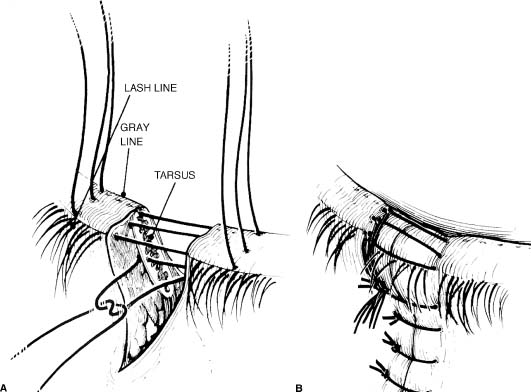
Eyelid Lacerations
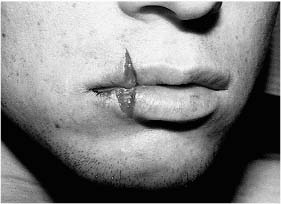
LIPS

NASAL LACERATIONS
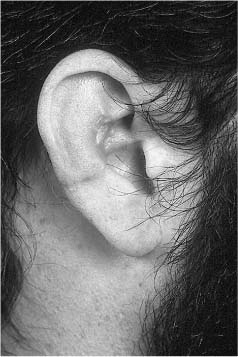
EXTERNAL EAR INJURIES
TREATMENT OF LACERATIONS OF THE EXTERNAL EAR
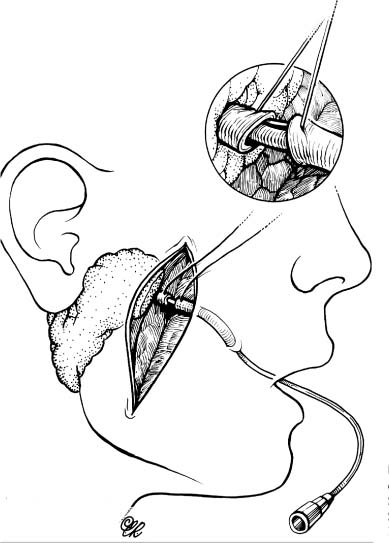
Parotid Duct Injuries
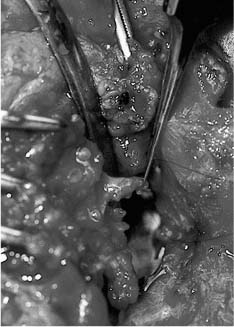
Facial Nerve Trauma
SOFT TISSUE INJURY FOLLOW-UP
FACIAL FRACTURES
BASIC PRINCIPLES
BONE HEALING
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree