Mandible Reconstruction With Scapula Flap
Mouchammed Agko
Hung-Chi Chen
DEFINITION
Reconstruction of the mandible requires bone to replace osseous defects and soft tissues to reconstruct the floor of mouth, tongue, soft palate, and/or external skin. This combined soft tissue and osseous reconstructive needs, in combination with vascular anatomy and other patient factors, determine the optimal donor site. In specific circumstances, the subscapular system of flaps can be employed for this purpose.
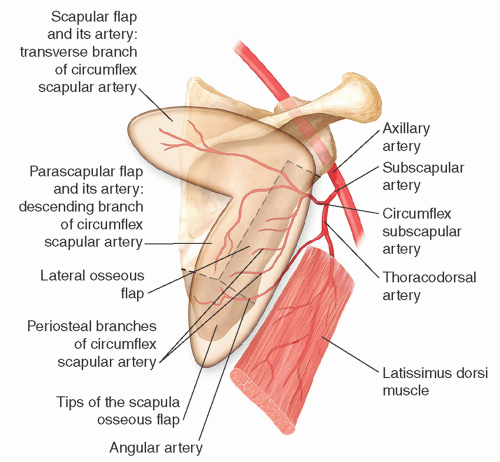
The subscapular vascular system is one of the most versatile and reliable donor sites with consistent anatomy that can provide a multitude of skin, fascia, muscle, and osseous flaps combinations.
Saijo and dos Santos laid the anatomic foundations for the scapular flap leading to the first successful microsurgical transfer by dos Santos, Gilbert, and Teot.1,2,3 Nassif subsequently described the parascapular flap.4
In 1981, Teot et al. reported use of an osseous scapular flap based on the circumflex scapular artery (CSA).5 Later, Swartz et al. popularized the use of the osteocutaneous scapular flap for mandibular reconstruction.6
Historically, it is interesting to note that although Ignio Tansini is credited for the first description of the latissimus dorsi flap, his initial depictions of the flap have been actually determined to be consistent with that of a parascapular flap.
ANATOMY
The subscapular artery (SSA) arises at the distal border of the subscapularis muscle from the third portion of the axillary artery.
It measures 2 to 4 mm in diameter and courses for 2 to 3 cm prior to dividing into the CSA and thoracodorsal artery (TDA).
The CSA, which is 1.5 to 3.5 mm in diameter, courses for 3 to 4 cm before it gives off the branches to the lateral border of the scapula.
It enters the triangular space (omotricipital triangle) that is formed by divergence of the teres muscles from the lateral scapula (teres minor superiorly, teres major inferiorly) and the long head of the triceps laterally.
At this level, it sends branches to the subscapularis muscle and numerous musculoperiosteal branches to the lateral border of the scapula.
Subsequently, it gives off branches to the infraspinatus and teres muscles.
It is the musculoperiosteal branches between the anterior subscapularis muscle and the posterior teres muscles that provide the vascularization of the upper two-thirds of the lateral scapula and have to be preserved during harvest of the lateral osseous flap.
The distance from the axillary artery to these branches (effectively the pedicle length of the flap) is 6 to 8 cm.
After emerging through the triangular space, the CSA is encased in the dorsal thoracic fascia.
An areolar space separates this from the underlying deep muscle fascia.
The CSA courses caudally for 2 to 3 cm before it divides into its terminal branches: ascending, transverse, descending, and anterior.
Although all these can serve as the source for axial flaps overlying the specific branch, the two most commonly used are the scapular and parascapular flaps based on the main transverse and descending branches, respectively (FIG 1).
The circumflex scapular system has two veins of disparate size that coalesce before joining the single vein of the thoracodorsal system to form the subscapular vein (SSV) (2.5-4 mm in diameter).
Anatomic variations:
Double CSA (8%-14%)
Double circumflex scapular vein (CSV) (14%)
CSA originating separately from the axillary artery (4%-8%)
CSV originating separately from the axillary vein (12%)
Descending branch of the CSA (parascapular flap pedicle) coursing anterior to the teres major muscle (TM) and emerging between the inferior border of the teres minor muscle (Tm) and the superior border of the latissimus dorsi muscle (7 of 30 cases)
SSA and thoracoacromial arteries sharing a common origin from the axillary artery (2-47 cases)
PATIENT HISTORY AND PHYSICAL FINDINGS
The shoulder and axillary area is carefully examined. Range of motion of the shoulder joint and strength of the parascapular muscles are determined.
Previous injuries and surgical interventions in the vicinity are scrutinized to assess potential damage to the vascular structures precluding safe harvest.
Although scars involving just the skin do not preclude harvesting the cutaneous portion of the flap, deeper scars (eg, previous thoracotomy incision, previous axillary lymph node dissection) may have injured the pedicle or the flap.
Special attention is paid to identify spinal accessory nerve injury by examining trapezius muscle bulk and function. Further compromise of parascapular muscles may exacerbate winging of the scapula.
IMAGING AND OTHER DIAGNOSTIC STUDIES
No preoperative studies are required.
In cases of previous injury or surgery, angiography (conventional, computed tomography, or magnetic resonance) can be employed to confirm intact vascular anatomy.
Theoretically, x-rays of the scapula or computerized tomography with 3D reconstruction could be used for preoperative planning.
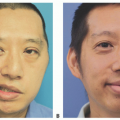
For the purposes of photographic documentation, preoperative photos of the donor site and shoulder function are obtained.
SURGICAL MANAGEMENT
The available reconstructive options for mandibular defects are as follows:
Osteocutaneous fibula flap
Vascularized iliac crest flap
Osteocutaneous scapula flap
Osteocutaneous radial forearm flap
Vascularized rib with blood supply from the latissimus dorsi muscle
Vascularized medial femoral condyle corticoperiosteal flap for short defects of the mandible
Reconstruction plate covered with pedicle or free myocutaneous flap
Delayed repair with nonvascularized bone graft
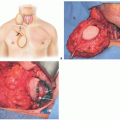
Anterior, sagittal, or hemimandibular defects can be restored with the osteocutaneous scapula flap.
Specific indications for the use of the osteocutaneous scapular flaps in mandibular reconstruction include the following:
Composite defects of the mandible with large soft tissue requirements for intraoral lining, external coverage, and/or soft tissue augmentation.
The soft tissue defect is not directly adjacent to the osseous defect and relative independence is required between the bone and soft tissue component for optimum inset.
Fibula is not available due to vascular disease, aberrant anatomy, injury, or previous harvest.
Patient wants to avoid the morbidity of vascularized iliac crest harvest.
Medical status prohibiting lengthy procedures under general anesthesia would preclude the use of this flap.
Relative contraindications include the following:
Osseous defect larger than 14 cm in men or 10 cm in women
Need for multiple osteotomies that could endanger viability of the osseous flap
Pre-existing shoulder disorders limiting range of motion and periscapular muscle strength
Damage to the vascular pedicle due to injury or procedure (eg, lymphadenectomy)
Skin of the back too thick (obese patients)
There are numerous advantages of this flap compared to other options for mandibular reconstruction.
The hairless nature of the scapular skin flap closely resembling the texture and sometimes color of the facial skin.
The vascular pedicle can be dissected to the appropriate length and diameter to match the recipient vessels.
Additional flaps can be carried on a single arterial and venous anastomosis except in cases of anatomical variations.
The soft tissue elements can be spatially oriented independently of each other or the bone enabling coverage of complex composite defects.
Primary closure of large skin flaps is possible providing more skin than other osteocutaneous flaps.
Limited morbidity with minimal loss of shoulder function if no preoperative limitation exists.
The vascular pedicle is reliable even in patients with diabetes or arteriosclerosis.
Scapular osteocutaneous flaps also have some limitations.
Some authors have described flap harvest in a modified semilateral position to avoid redraping. However, most surgeons prefer to reposition the patient before and after flap harvest.
Two-team approach is not possible. Mandibular resection/recipient site preparation has to be completed prior to proceeding with the flap harvest.
Revascularization of the flap can only be performed after donor-site closure.
If undue tension is present at closure, the donor-site scar may be widened. However, patients rarely complain as the scar is not easily visible to them.
If skin grafting becomes necessary, this is an inherently unfavorable site.
Dissection of the CSA pedicle through the triangular space can be tedious and time-consuming.
Because the sensory branches from the 3rd to 5th intercostal nerves are divided during elevation of the flap, primary sensory innervation is not possible. However, when transferred to the head and neck region, the flap may regain satisfactory sensation without nerve repair through spontaneous regeneration from the tissue bed.
Bone stock may be limited in women precluding dental rehabilitation.
Preoperative Planning
For preoperative marking and flap design, the patient is seated or stands comfortably with the arms resting at the sides.
The borders of the scapula and its spine are marked.
The triangular space is identified by palpating the lateral border of the scapula at 2/5th of the distance between the center of the spine and the inferior angle of the scapula.
The posterior axillary fold formed by the teres major (TM) is pinched between the thumb and the remaining fingers to identify the upper border of the muscle.
The emergence point of the CSA from the triangular space can be located at area where the upper border of the TM meets the lateral border of the scapula.
A pencil Doppler can confirm the location of the CSA and the course of its branches.
Scapular fasciocutaneous flap
The axis of the scapular flap is a line parallel to the spine of the scapula extending from the emergence point of the CSA to the posterior midline.
The flap itself is marked from the posterior axillary line to the posterior midline in a lenticular fashion with the medial end extending over the triangular space. The design is limited superiorly by the spine of the scapula and inferiorly by the inferior angle.
An additional 2 cm passing across the midline can be included as an extension based on random perfusion.
Usual flap dimensions allowing primary closure are 20 cm × 10 cm, with the length ranging from 6 to 25 cm and the width from 4 to 16 cm.
Medial osseous flap
The scapular fasciocutaneous flap can carry an osseous segment from the medial border of the scapula (10-12 cm × 3 cm × 0.71-1.5 cm) through its fascial attachments to the bone.
Parascapular fasciocutaneous flap
The axis of the flap corresponds to the lateral border of the scapula when the arm is in adduction.
The flap is marked from the posterior axillary crease superiorly and can extend to the 12th rib or midway between the inferior angle of the scapula and posterior superior iliac spine.
The width that would allow primary closure is confirmed by pinching the skin and subcutaneous tissue.
Usual flap dimensions allowing primary closure are 24 cm × 12 cm, with the length ranging from 6 to 32 cm and the width from 8 to 20 cm.
Lateral osseous flap
The length of the lateral scapular bone that can be reliably elevated on the periosteal branches of the CSA depends on number of planned osteotomies.
Usually up to 10 cm in women and 14 cm in men can be harvested, when one proximal osteotomy is planned.
The bone can be up to 3 cm wide with variable thickness (1.5-3 cm) along the length of the flap.
If more and/or distal osteotomies or longer bone is needed, the scapular tip (an additional 3 to 4 cm of bone) can be safely and simultaneously harvested based on the angular artery. Alternatively, the tip of scapula can be harvested as a separate vascularized bone segment (FIG 2).7
The angular artery branches off the TDA or the serratus branch and travels on top of the serratus muscle beneath the TM.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree