Fig. 22.1
Five basic types of perianal fistula, namely, superficial (1), intersphincteric (2), transsphincteric (3), suprasphincteric (4), and extrasphincteric (5) (Reproduced with permission from Hughes [30])
In 1992, Hughes [30] proposed a clinical classification based on anatomic and pathologic aspects derived from a 20-year prospective study of anal Crohn’s disease in Cardiff, UK. The main classification defines the presence of ulcerations, fistulae or abscesses, and strictures qualified by numeric values reflecting severity: 0 = not present, 1 = limited clinical impact, and 2 = severe. A subsidiary classification defines associated conditions (such as hemorrhoids and supervening cancer), proximal intestinal involvement (enteric and colonic), and disease activity (inactive vs. active) (Tables 22.1 and 22.2).
Table 22.1
Hughes-Cardiff classification
Ulceration (U) | Fistula/abscess (F) | Stricture (S) |
|---|---|---|
0 Not present | 0 Not present | 0 Not present |
1 Superficial fissures | 1 Low superficial | |
Fleshy skin tags | Anovulval/scrotal | |
Intersphincteric | ||
Anovaginal | ||
2 Cavitating ulcers | 2 High | 2 Irreversible stricture |
Anal canal | Blind supralevator | Anal stenosis |
Rectum | Rectovaginal | |
Confluent skin ulcers | Ileoperineal | |
Associated anal disease (A) | Proximal disease (P) | |
Hemorrhoids | 0 None | |
Malignancy | 1 Contiguous rectal disease | |
2 Colon (rectal sparing) | ||
3 Small bowel |
Table 22.2
Simple Hughes classification
Ulceration (U) | Fistula/abscess (F) | Stenosis (S) |
|---|---|---|
0 Not present | 0 Not present | 0 Not present |
1 Superficial fissure | 1 Low | 1 Membranous |
2 Cavitating | 2 High | 3 Fibrotic |
A technical review from the American Gastroenterological Association in 2003 proposed a classification system [31] with just two categories: simple and complex fistulae. Simple fistulae are low (i.e., superficial, low intersphincteric, or low intrasphincteric) with a single external opening and are not associated with perianal abscesses, the vagina or bladder, rectal stenosis, or macroscopic proctitis. In contrast, complex fistulae are high (i.e., high intersphincteric, high intrasphincteric, suprasphincteric, or extrasphincteric), can have several external openings, and may be associated with perianal abscesses, the vagina or the bladder, rectal stenosis, or macroscopic proctitis.
Irvine [32] developed the Perianal Disease Activity Index (PDAI) to measure morbidity associated with perianal disease. This index assesses five categories related to fistulae, including discharge, pain, restriction of sexual activity, the type of perianal disease, and the degree of induration. The PDAI assesses various aspects of quality of life that are most affected in patients with perianal disease. Table 22.3 represents a modified Irvine classification.
Irvine classification | |||||
|---|---|---|---|---|---|
Discharge | None | Minimal mucus | Purulent | Fecal | |
Pain | None | Mild | Moderate | Severe | |
Sexual restriction | None | Slight | Moderate | Severe | |
Type of perianal disease | |||||
None | Fissure | >3 Fistulae | >3 Fistulae | Ulcers | |
Degree of induration | None | Minimal | Moderate | Extensive | |
Abscess | |||||
Diagnosis/Evaluation
Many manifestations of anorectal Crohn’s disease are readily observed during a thorough physical examination. A tissue biopsy documenting noncaseating granulomas is only occasionally necessary; however, a complete assessment of the extent of intestinal as well as perianal pathology is needed before any management decisions can be made. Diagnostic efforts frequently are focused on defining the type, location, and nature of perianal involvement. This evaluation usually entails a detailed history and physical examination and a combination of endoanal ultrasound (EAUS), magnetic resonance imaging (MRI) of the pelvis, an examination under anesthesia (EUA), or all three. An EUA traditionally has been considered the gold standard. In a prospective study by Schwartz and colleagues [33] that included 34 patients, it was found that EUA alone had an accuracy of only 90 % compared with EUA combined with anal EAUS and pelvic MRI. This same study found that the diagnostic accuracy was 100 % when any two of the three procedures were performed.
EAUS provides detailed visualization of the anal sphincter complex. On a normal ultrasound, the internal sphincter, intersphincteric space, and external sphincter are visible as concentric circular layers. The internal sphincter is hypoechoic and 2–3 mm in width. The intersphincteric space is echogenic, whereas the external anal sphincter has a mixed echogenicity pattern. A tract leads to disturbance of normal anatomy, often visible as a hypoechoic linear structure, although echogenic air bubbles can be present in some cases. Accuracy rates for correctly classifying the fistula tract are between 86.5 [34] and 95 % [35]. In a larger patient series, the internal opening was detected in 62.5–94 % of cases [36, 37]. The inconsistency in the literature concerning the accuracy of EAUS for perianal fistula can be explained partially by those reports utilizing the installation of hydrogen peroxide and in cases where echogenic shadowing across an intersphincteric abscess may overcall certain fistulae as unduly high in locale. It has been well established that hydrogen peroxide improves visualization of the fistula tract [34, 35, 38–43]. With the use of three-dimensional reconstruction of two-dimensional images, some investigators have shown that the results of EAUS are comparable with those of MRI and are performed with excellent patient tolerance [44, 45]. In this respect, recent data has suggested that postprocessing of EAUS three-dimensional images, which alters the image opacity, luminance, thickness of beam penetration and image filtration by removing low-intensity pixels from the data set, enhances the preoperative definition of abscesses and internal openings [46]. These volume-rendered modes recommend low thickness, low filter, mid-luminescence, and mid- to low opacity parameters. One small study using hand-held transperineal sonography in comparison with operative findings in non-Crohn’s anal fistulae has shown high accuracy for definition of type and for the determination of the location of the internal opening, although this is weakened if there is significant horseshoeing or an associated gas-containing abscess [47]. There was no benefit of hydrogen peroxide instillation.
Pelvic MRI has been shown to have an accuracy of 90 % when classifying fistulae and 97 % when delineating complex abscesses [48, 49]. Moreover, surgical management may be altered in 10–20 % of patients by the addition of MRI to EUA, and this increases to up to 40 % in patients with Crohn’s disease [50, 51]. In patients with complex perianal Crohn’s disease, therefore, it is a common practice to combine a pelvic MRI with EUA and proctoscopy to evaluate for coincident rectal inflammation. The use of endoanal MRI, which utilizes a hemiacetal polymer rectal probe linked to the external magnet, has shown high accuracy in the delineation of the internal opening close to the probe, although there is limited information in Crohn’s disease [52, 53]. This device is not widely available and suffers from the limitations of any endoluminal technology in that lateral extrasphincteric fistulae beyond the focal distance of the probe and supralevator extensions where the probe is unable to adequately couple above the puborectalis are not well demonstrated. A recent development is the use of dynamic contrast-enhanced MRI for determining disease activity in perianal Crohn’s disease [54]. Using this technique, two-dimensional T1-weighted scans are performed and time intensity curves with contrast enhancement are obtained so that fistula “activity” can be determined. In patients with active fistulae, the volume of enhancing pixels is higher than that in patients with inactive fistulae [54].
Fistulography has been shown to have an accuracy of 16–50 % [55, 56] and is not routinely recommended. Although many reports have described its accuracy in the delineation of an internal opening, it is painful, may be associated with significant bacteremia, and the relationship of the fistula track to the main sphincter complex can be only inferred. Likewise, computed tomography (CT) has proved to be relatively unreliable in assessing perianal disease; its accuracy ranges from 24 to 60 %, [57–63] partially because the volume-averaging effects of the technology do not provide sufficient resolution between fistula tracks and ischiorectal fat.
Once anorectal disease is delineated, evaluation for proximal Crohn’s disease with endoscopy and small-bowel radiography should be considered. A study of 5,491 patients with Crohn’s disease found an association between proximal fistulizing disease and perianal fistulae [64]. Some investigators have found that treatment of proximal disease may assist in the resolution of anorectal symptoms [2, 65]; however, other investigators did not observe improvements in their series [66, 67]. Most experts do not recommend operative intervention on the proximal bowel solely to improve anorectal disease [68, 69].
Management
In view of the wide spectrum of perianal lesions and associated symptoms, treatment is often variable and individualized. A multidisciplinary approach is essential for the successful management of patients with complex disease, and the close collaboration of the surgeon and the gastroenterologist is essential in the management of these patients. Treatment may vary depending on the severity of the symptoms. Other considerations include the nutritional status of the patient and the extent and severity of the disease in the gastrointestinal tract. The concerns and expectations of the patient must also be considered in the decision making process. In patients who have relatively few symptoms despite what appears to be severe perianal disease, the goal should be minimization or alleviation of their symptoms rather than eradication of their disease and avoidance of proctectomy, although proctectomy may be inevitable in 20 % of patients [10]. Although treatment may vary according to the specific lesions in the individual patients, certain general measures such as improvement in nutritional status, treatment of the proximal disease, and local skin measures including sitz baths or showers and the use of local barrier creams are of benefit to most patients.
Spectrum of Disease
Perianal Crohn’s disease encompasses nonfistulating lesions such as skin tags, hemorrhoids, ulcerations, strictures, and cancer as well as fistulating lesions including fistulae, abscesses, and rectovaginal fistulae.
Skin Tags
In a combined medical and surgical Crohn’s disease follow-up clinic, Keighley and Allan [70] reported the presence of anal skin tags in 375 patients with Crohn’s disease and in 68 % of the subgroup of patients with Crohn’s disease and perianal disease. Although skin tags are commonly found in patients with Crohn’s disease limited to the colon, about 36.7 % of patients with anal skin tags have ileal involvement [71]. Two types of skin tags are described here [72]. The first is the typical Crohn’s disease skin tag, which is large, edematous, hard, and cyanotic. These skin tags arise from lymphedema secondary to lymphatic obstruction and often coexist with intestinal inflammation. The second type is a flat and broad- or narrow-based lesion often referred to as an “elephant ear” tag. These tags are soft and painless.
Skin tags are generally persistent but benign. They often become enlarged and edematous with coexisting intestinal inflammation. Only one case of malignant transformation has been reported in the literature [73]. In a series of 109 patients referred to a tertiary center, 68 % of the patients who had skin tags at diagnosis still had them 10 years later [74]. Of these patients, 86 % were asymptomatic [75]. Skin tags may increase in size and thickness and may become firmer during an active Crohn’s disease flare [76].
Management
Anal skin tags are innocuous and usually managed expectantly unless they interfere with hygiene or are persistently symptomatic [70] because there is an increased risk for delayed wound healing.
Hemorrhoids
Hemorrhoids occur infrequently in patients with Crohn’s disease and are seen in only 7 % of cases [70], which is lower than the estimated prevalence of 24 % in general population [77]. Although often asymptomatic, symptoms can be exacerbated by severe diarrhea associated with Crohn’s disease.
Management
Surgical intervention is generally avoided because of the significantly high rate of delayed wound healing, inflammation, infection, and stenosis [78]. The perceived conservative management of symptomatic hemorrhoids in patients with Crohn’s disease has come from one report of a retrospective series of a small number of cases reported by the St. Mark’s group in 1977, where poor wound healing was noted and resulted in half the patients undergoing proctectomy. This series extended over a long period, however, and therefore the nature and conduct of hemorrhoidectomy should be viewed with some skepticism, and there was no clear evaluation of the indication for subsequent rectal excision. In a highly selected group of patients without any evidence of rectal Crohn’s disease, however, hemorrhoidectomy can be safe and useful in up to 88 % of symptomatic patients [79]. The role for other less extensive procedures designed to manage hemorrhoids, including rubber band ligation, Doppler-guided hemorrhoidal dearterialization, and stapled procedure for prolapse and hemorrhoids/hemorrhoidopexy, is at present unclear.
Fissures and Ulcers
Ulcerations in Crohn’s disease are divided into fissures and ulcerations in accordance with the simplified Cardiff classification. The reported incidence of anal ulcerations in Crohn’s disease is 43 % [12, 80–83]. Superficial fissures constitute 21–35 % of all Crohn’s-related anal fissures [80, 84]. Cavitating ulcers occur with an incidence of 5–10 % [12, 70, 85].
Ulceration
Although anal ulcerations associated with Crohn’s disease are classically described as painless, pain has been reported in up to 70 % of patients [82, 86, 87]. Other symptoms include discharge, pruritis, and bleeding. Cavitating ulcers are likely to lead to major fistulous tracts. The edges of the ulceration are often edematous, irregular, undermined, and detached. Cavitating ulcers may occur in the upper anal canal or adjacent to the rectal mucosa. Extension of the ulcerations outside the anal canal to the perianal skin is rare but may occur in the acute aggressive form of the disease [29]. Concomitant proctitis is present in 75–96 % of cases [85, 88]. Multiple ulcerations have been observed in 14–33 % of patients with ulceration [81–83]. Ulcerations can have two distinct evolution patterns: Many of them heal spontaneously, whereas in some cases they can lead to the formation of a fistula, an abscess, or anal stenosis. The long-term outcome of cavitating ulcers is usually poor, with a risk of proctectomy reported in up to 83 % of patients [70]. An example of extensive ulceration is shown in Fig. 22.2.
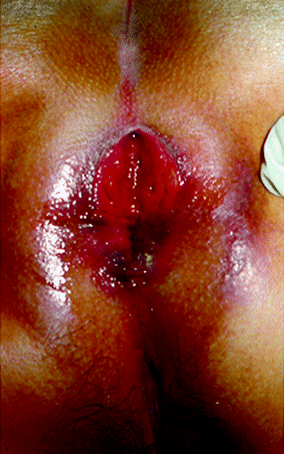

Fig. 22.2
Perianal ulceration
Fissures
Fissures in Crohn’s disease are thought to result from direct ulceration due to the disease process and may not necessarily be related to internal sphincter spasm, as is the case in idiopathic fissures. In contrast to idiopathic anal fissures. which are almost always located in the midline, fissures in patients with Crohn’s disease can be eccentrically located in up to 20 % of patients and are often multiple. A Crohn’s-related anal fissure extending into the perianal skin is shown in Fig. 22.3, with marked differences when compared with the appearance of an idiopathic anal fissure.
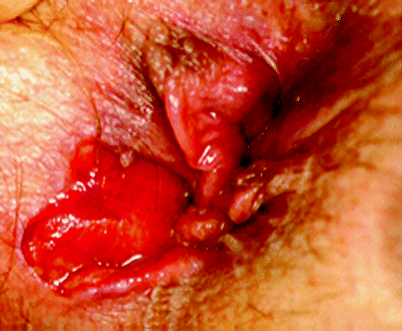

Fig. 22.3
A Crohn’s-related anal fissure
Medical Management
Topical agents have been used to decrease symptoms but do not promote healing of lesions. Metronidazole (10 %) ointment has not been shown to be effective in the reduction of the PDAI score, but some secondary outcomes such as pain and discharge have shown improvement, suggesting treatment effect [89]. A small case series of five patients and a randomized, controlled trial of seven patients showed that tacrolimus (0.5–1 mg/g) did not heal ulcerations but resulted in rapid improvements in terms of depth, surface area, induration, and pain in all patients [90]. Local intralesional corticosteroid therapy can be effective in some patients as well.
Systemic agents also have been tried for the treatment of anal ulcers, with some efficacy, but their side effects usually preclude their use. In a recent retrospective study of 99 patients, infliximab therapy was shown to be well tolerated and effective in inducing and maintaining a complete clinical response for anal ulcerations [91]. After infliximab induction therapy, 42.5 % of patients with ulceration had a complete response. Long-term results after a median follow-up of 175 weeks showed a complete response in 72 % of patients with ulceration, with broadly similar results for superficial fissures and cavitating ulcers. Infliximab is accompanied by a rapid resolution of symptoms and the benefit is sustained over time. Overall, recent evidence supports the use of anti-tumor necrosis factor (TNF) therapy in the treatment of symptomatic ulcerations. Several reports have suggested the benefit of hyperbaric oxygen and an elemental diet in promoting the healing of anal ulceration and fissures [92–95]. The role of medical agents such as topical nitroglycerine, calcium channel blockers, and botulinum toxin, which have been shown to be effective in the treatment of idiopathic fissures in Crohn’s disease, is unknown [96].
Surgical Management
Fortunately, fissures in people with Crohn’s disease typically display a self-limiting course: only 10 of 53 patients (19 %) in one series still were affected after 10 years of follow-up [96]. Patients with painful refractory fissures can in some cases undergo lateral internal sphincterotomy once sepsis has been excluded. The rate of healing after surgery is high with a low incidence of complications. Fissures that remain unhealed have a propensity to develop into a perianal abscess or fistula or both, and proctectomy often will ultimately be required. Local anorectal procedures, particularly a lateral internal sphincterotomy, should be used judiciously in patients who do not respond to conservative treatment. If sphincterotomy is contemplated, consideration should be given to a closed technique to limit the size of the potentially unhealed wound [83]. Symptoms secondary to large cavitating ulcers often can be controlled with debridement of the overhanging edges and intralesional steroid injection. Nevertheless, some of these patients may eventually require proctectomy because of unrelenting pain, sepsis, or fecal incontinence.
Strictures
Anal or rectal strictures may arise as complications of ulceration of the anal canal or rectum, perianal abscesses, and perianal fistulae and often are associated with ongoing rectal inflammation, complex perianal fistulae, and rectovaginal fistulae. A minor degree of stenosis of the anal canal is frequently encountered in patients with perianal Crohn’s disease. Referral centers have reported a variable incidence of anal strictures, from 9 to 22 % [97, 98]. The pathology of strictures differs between the anus and the lower rectum, accounting for 34 and 50 %, respectively, of anal strictures in perianal/perirectal Crohn’s disease. Strictures can be short anal strictures or long tubular strictures involving varying lengths of the rectum. The presence of colonic disease and an anal canal stricture is significantly associated with the need for a permanent stoma [99].
Management
Anal dilatation is currently performed to treat symptomatic anal stricture by gentle digital dilatation or by using a coaxial balloon technique [87]. Initially this should be performed extremely gently under anesthesia. Dilatation should be performed judiciously because of the risk of sepsis: the risk is increased in the event of severe proctitis or associated sepsis encountered during surgery. Patients with severe strictures who fail to respond to repeat dilatations may require proctectomy [96].
Abscess and Fistula
Pyogenic complications are commonly encountered in patients with perianal Crohn’s disease. These usually present in the form of an abscess or a fistula. The reported frequency of perianal fistula in Crohn’s disease varies from 17 to 43 % of the Crohn’s disease population [100]. The complexity of fistula that follows depends on the type of abscess drained. Patients with abscesses frequently present with an acute onset of pain and require urgent surgical intervention. Fistula can present as recurrent abscesses with or without spontaneous drainage, continuous discharge, pain, and occasionally incontinence. The etiopathogenesis of perineal fistula in Crohn’s disease is not entirely understood, but it is generally believed that they are either a direct consequence of penetration and undermining of perianal ulcers from fecal material or as a result of abscess formation. Defects in fibrinogenesis and matrix metalloproteinase expression also are thought to contribute to the development of perianal fistula in Crohn’s disease [101].
Perianal Crohn’s disease cannot be cured by medical or surgical treatment. The aim of therapy is to alleviate symptoms and treat complications of the disease to improve the patient’s quality of life. Principles guiding therapy involve the ability to clear the area of sepsis, avoiding damage to the anal sphincter, and medical management to assist the achievement of definitive surgical treatment.
Abscess/Perianal Sepsis
Abscesses can be present in any plane (superficial, intersphincteric, ischiorectal, or supralevator), but regardless of their location, they require prompt surgical incision and drainage and treatment of systemic symptoms with broad-spectrum antibiotics. Pain, swelling, and fever are the hallmarks of an abscess. The patient with a supralevator abscess may complain of gluteal pain. Rectal bleeding also has been reported. Severe rectal pain accompanied by urinary symptoms such as dysuria, an inability to void, and urinary retention may suggest an intersphincteric or supralevator abscess.
Inspection will reveal erythema, swelling, and possible fluctulance with perianal or ischiorectal abscesses. It is crucial to recognize that with either intersphincteric or supralevator abscesses, no visible external manifestations are present despite the patient’s complaint of excruciating pain. With a supralevator abscess, a tender mass may be palpated during rectal or vaginal examination. The presence of a black spot may be indicative of a widespread necrotizing infection.
Management
For superficial abscesses, a simple incision and drainage procedure is effective. Incision (elliptical or cruciate) is made on the medial aspect of the overlying skin. The surgeon should attempt to minimize the amount of tissue opened if a fistula is found once the inflammation subsides. Most ischiorectal abscesses can be incised and drained in a similar fashion; however, horseshoe abscesses should be drained with the patient under either regional or, preferably, general anesthesia. An opening is made in the posterior midline and the lower half of the internal sphincter is divided to drain the postanal space–this is believed to be the source of infection. Counter incisions are made over each ischiorectal fossa without wide incisions to allow drainage of the anterior extensions of the abscess. Because a diagnosis of an intersphincteric abscess is considered when the patient presents with pain out of proportion to the physical findings, an EUA is mandatory to thoroughly assess the cause of the pain. Once the diagnosis is made, the internal anal sphincter is divided along the length of the abscess cavity.
Before a supralevator abscess is drained, its origin should be determined: It may arise from an upward extension of an intersphincteric abscess, an ischiorectal abscess, or a downward extension of a pelvic abscess. If its origin is an intersphincteric abscess, it should be drained through the rectum and not through the ischiorectal fossa because the latter will result in a suprasphincteric fistula. However, if it arises from an ischiorectal abscess, it should be drained as such and not through the rectum because rectal drainage will result in the creation of an extrasphincteric fistula. If the abscess is of pelvic origin, it can be drained through the rectum, ischiorectal fossa, or abdominal wall, depending on the direction at which it is pointing, although this has the risk of creating an extrasphincteric fistula, which can be difficult to treat effectively. With deep or high abscesses (supralevator or ischiorectal), it is more likely that the fistula tract or the surgical incision made to drain the abscess may become obstructed, requiring the placement of a mushroom catheter or a noncutting seton is required to ensure continuous adequate drainage. This can be effective in supralevator extensions from primary ischiorectal abscesses with attendant drain shortening. The external portion of the catheter is shortened to leave 2–3 cm outside the skin when the tip is in the depth of the abscess cavity. This reduces the chances that the catheter will fall out of or into the cavity in certain circumstances, and it can be a long-term solution for complex fistulae, especially if active proctitis is present.
The need to identify the internal opening at the time of initial abscess drainage is controversial. Most studies suggest that an aggressive search is not warranted. For recurrence, a search for the concomitant fistula is necessary after resolution of the active inflammation. A simple method during EUA utilizes hydrogen peroxide or diluted methylene blue injected into mushroom catheter or via the external opening. In patients with persistent sepsis or disease progression despite initial abscess drainage, a temporary diverting stoma can be effective, creating a favorable environment in which to perform local perianal repair. In this respect, the laparoscopic approach is now preferred because it is minimally invasive and allows the opportunity to assess the presence of associated proximal intestinal disease
Fistula
Figure 22.4 shows an EAUS of a transsphincteric fistula track.
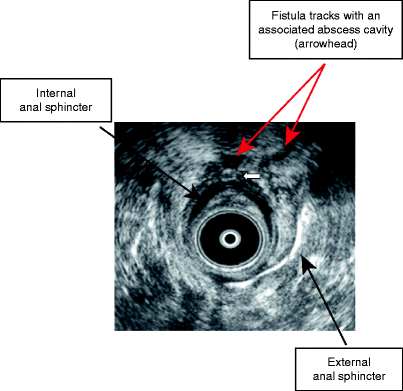

Fig. 22.4
Endoanal ultrasound of a transsphincteric fistula track
Medical Management
Antibiotics
Bacteria may, in theory, play a role in the appearance and persistence of perianal fistulous disease. Thus, antibiotics are sometimes used as first-line therapy for fistula healing. In other cases, antibiotics, in view of their prophylactic effects against infections and abscesses, are used as adjuvant (or bridging) therapies. Most of the studies of perianal fistulating disease treated with antibiotics are uncontrolled and the sample sizes are small. In these studies, metronidazole [102–104] and ciprofloxacin [105], as well as a combination of the two drugs [106], have shown an initial beneficial effect on perianal fistulae. Response typically occurs after a 6- to 8-week treatment schedule and is usually manifest in the form of decreased drainage. Fistula closure is uncommon, however, and symptoms tend to recur after treatment has been suspended [104].
Metronidazole is typically commenced at a dose of 20 mg/kg/day divided into three doses, usually totaling 1,000–1,500 mg/day. The side effects of metronidazole include nausea, a metallic taste, and peripheral neuropathy in the form of troublesome paraesthesia. Side effects are often severe enough to necessitate dose reduction or discontinuation of the medication. Bernstein et al. [102] reported a series of 21 patients with perianal Crohn’s disease treated with metronidazole. All patients had a clinical response, with a decrease in pain and tenderness. Fifty-six percent had complete healing of their perianal disease. Clinical improvement typically occurred after 6–8 weeks of therapy. A follow-up study by the same group [103] showed exacerbation of disease with dosage reduction, as well as the occurrence of paresthesiae in 50 % of patients.
Ciprofloxacin is typically used at a dose of 1,000 mg/day (500 mg bid), either alone or in combination with metronidazole. Similar studies have been reported by others [104]. A recent study by West et al. [105] compared the combination of infliximab with either ciprofloxacin or placebo in 22 patients. The follow-up, although short, showed at 18 weeks that patients treated with ciprofloxacin tended to respond better than the placebo group (1⁄4 2.4). The response was measured both clinically (as a 50 % reduction in the number of draining fistulae) and radiologically (utilizing three-dimensional EAUS and hydrogen peroxide injection). Side effects from ciprofloxacin are rare and include headache, nausea, diarrhea, and rash [106]. A similar study by Solomon and colleagues [107] showed a similar advantage for combination therapy without preliminary infliximab.
Thiopurines
Azathioprine (AZA) and 6-mercaptopurine (6-MP) have shown efficacy in the treatment of Crohn’s perianal fistulas. In a meta-analysis of five controlled studies, a response (defined as complete closure or decreased drainage) was found in 54 % of the patients treated with AZA or 6-MP compared with 21 % in the placebo group (odds ratio, 4.44; 95 % confidence interval, 1.50–13.2) [108]. This meta-analysis is limited in that fistula response was a secondary endpoint and not the primary measurement parameter in all of the studies included. There have been no controlled trials in which the primary endpoint was assessment of the effect of thiopurines on the closure of fistulae in patients with Crohn’s disease. The initial dose of AZA is 2.0–3.0 mg/kg/day and for 6-MP is 1.0–1.5 mg/kg/day. Regular monitoring of complete blood counts and liver enzymes must be performed if these medications are commenced. Because of their prolonged onset of action, these medications are often started in conjunction with other agents such as antibiotics or infliximab.
Immunosuppressant Therapy
Cyclosporine and tacrolimus are calcineurin inhibitors that act by abrogating T-cell activation and interleukin (IL)-2 production. Both agents possess potent immunomodulating properties and have been successfully used in the prevention of solid organ rejection for decades. Multiple uncontrolled trials have documented the efficacy of cyclosporine in the treatment of perianal disease, with closure rates around 90 % and a rapid duration of action. In a study by Present and Lichtiger [109], intravenous cyclosporine was used at a dose of 4 mg/kg/day and then converted to 6–8 mg/kg/day per os. Recurrence rates were high once the treatment was discontinued. Tacrolimus at a dose of 0.2 mg/kg/day was given to 20 patients with perianal fistulae in a randomized, placebo-controlled trial by Sandborn et al. [110]. The medication was given for 10 weeks, with a primary goal of fistula closure of 50 % and maintenance of that closure for at least 4 weeks. Tacrolimus (per os) was found to be effective in inducing improvement in fistula drainage (43 % vs. 8 %) but not fistula remission (10 % vs. 8 %). Both medications are associated with significant side effects, particularly renal impairment. Patients require frequent monitoring of drug levels and renal function.
Monoclonal Antibody Therapy
TNF-α is a key inflammatory mediator involved in the pathogenesis of Crohn’s disease. A variety of anti–TNF-α antibody therapies are available for the treatment of Crohn’s disease, including certolizumab pegol (Cimzia, UCB Pharma, Smyrna, GA), adalimumab (Humira, Abbott Laboratories, Abbott Park, IL), and infliximab (Remicade, Centocor, Malvern, PA). Currently, infliximab remains the most widely used anti–TNF-α antibody therapy in the treatment of Crohn’s disease. Infliximab is a genetically engineered chimeric monoclonal immunoglobulin (IgG) directed against soluble and transmembrane human TNF-α. TNF-α is a proinflammatory cytokine and is produced mainly by monocytes, macrophages, and T lymphocytes. It has important proinflammatory and immunostimulatory functions, including leukocyte activation, neutrophil chemotaxis, apoptotic inhibition of inflammatory cells, and the induction of other proinflammatory cytokines including IL-1, IL-6, and IL-8. In Crohn’s disease, levels of TNF-α as well as TNF-α–producing cells within inflamed bowel are increased, perpetuating inflammation. The actions of infliximab are not fully understood. Alongside neutralization of soluble and transmembrane TNF-α, infliximab may have other important functions such as inducing apoptosis in monocytes and lamina propria T cells after antibody binding to transmembrane TNF-α, as well as inhibiting neutrophil chemotaxis. Recently, infliximab has been shown to stimulate myofibroblast migration, which is particularly relevant in perianal fistula healing.
In a double-blind, randomized, controlled trial, Present et al. [111] compared two doses of infliximab—5 and 10 mg—with placebo at weeks 0, 2, and 6. The primary goal was fistula closure in 50 % from baseline observed at two or more consecutive study visits, and a secondary goal was closure of all fistulae. Sixty-eight percent of patients who received the 5-mg/kg dose achieved the primary goal and 55 % achieved the secondary goal compared with 26 and 13 %, respectively, in the placebo group. Patients receiving 10 mg/kg did not have an improved response over those who received 5 mg/kg, and both had a median response time of 2 weeks. Eleven percent of patients treated with infliximab developed perianal abscesses in the course of their treatment compared with 3 % in the placebo group. It is postulated that rapid closure of the external opening before closure of the tract leads to this complication. The ACCENT trial examined the role of maintenance therapy with infliximab, studying 306 patients with fistulizing Crohn’s disease who were treated with 5 mg/kg of infliximab at weeks 0, 2, and 6. One hundred ninety-five responders (69 %) were randomized to maintenance therapy with placebo or infliximab at 5 mg/kg every 8 weeks. The median time to loss of response was 14 weeks for placebo and more than 40 weeks for the infliximab group, which was a highly significant difference. Combining infliximab with AZA or 6-MP may result in longer-lasting remission and improved tolerance [112].
In general, once a fistula is diagnosed and sepsis is controlled, infliximab can be commenced at a dose of 5 mg/kg. It is administered on an outpatient basis in the hospital at weeks 0, 2, and 6. It is recommended that setons be removed at 2–6 weeks (between the second and the third dose) after the fistula has ceased draining. Patients who do not improve with the first two infusions are unlikely to respond after that, and the treatment should be discontinued. Moreover, despite the impressive clinical healing rate reported with infliximab, complete radiological closure may not necessarily follow [106, 113, 114], calling into question whether these fistulae will eventually reopen once the treatment is discontinued. Most reported adverse events, including headaches, nausea, upper respiratory tract infections, abdominal pain, and fatigue, are mild and not necessarily related to infliximab. Major side effects are rare and include pneumonia, fever, and dyspnea. There is an increased risk of opportunistic infections including miliary tuberculosis, sepsis, and Pneumocystis carinii pneumonia. There are also reports of an increased risk of lymphoma and heart failure.
Recently, localized delivery of infliximab has been proposed for the treatment of perianal fistulae in Crohn’s disease. This has the advantage of increasing the efficacy of treatment as well as minimizing the adverse effects and costs associated with systemic therapy. Two small studies have assessed the safety and efficacy of localized injections into fistulous tracts, with encouraging results. Lichtiger [115] reported four of nine patients who had complete healing of fistulae at 4 weeks, with a further three having a partial response. There were no reported adverse effects and no autoantibodies detected after 6 months. Similarly, Poggioli et al. [116] reported healing in 10 of 15 patients with no major side effects. These initial findings need further corroboration to determine the efficacy of a localized delivery approach. Despite the clinical appearance of fistula closure after medical treatment, MRI and endosonographic analyses reveal that the majority of fistula tracts persist. This explains the recurrence of fistulae in some cases after the withdrawal of the medical agent and suggests the need for long-term use of the agent to remain free of fistulae. However, because of the side effects and high costs associated with many of the drugs, long-term medical treatment is not feasible.
Miscellaneous Therapies
A pilot open study provided data suggesting that granulocyte macrophage colony stimulating factor (GM-CSF) is a safe and potentially effective agent for the treatment of active perianal Crohn’s disease [117]. GM-CSF has been used in a placebo-controlled study of patients with luminal Crohn’s disease, some of whom had draining fistulas at study entry. At 6 months, four of eight patients in the GM-CSF group and two of five in the placebo group had complete fistula closure [118]. There are not sufficient data to support the use of an elemental diet or oral spherical adsorptive carbon [119] in patients with Crohn’s perianal disease.
Summary of Medical Management
In the American Gastroenterological Association technical review [30], infliximab is recommended for the treatment of complex perianal disease, along with AZA or 6-MP and antibiotics for the induction phase. Maintenance is recommended with AZA or 6-MP in association with infliximab in some cases.
Surgical Management
Surgical treatment for perianal fistulas in patients with Crohn’s disease is based on the presence or absence of pancolitis and the location and type of fistula. Treatment should be individualized for each patient based on the surgeon’s experience and judgment. The quality of tissues within the rectum and the presence or absence of proctitis often dictates the appropriate surgical management.
The surgical procedures used to treat perianal fistulae remain largely unchanged and can still result in further morbidity. Surgical treatment of complex fistulae, such as high transsphincteric and suprasphincteric fistulae, is more likely to result in postoperative complications, particularly fecal incontinence or stricturing of the anal canal. Laying open complex perianal fistulae has been found to result in incontinence in more than 50 % of cases [120]. Sphincter-conserving techniques including core-out fistulotomy, permanent seton placement, and advancement flaps have been developed for complex fistulae; however, these procedures have high rates of recurrence.
Fistulotomy/Fistulectomy
Fistulotomy, also known as the laying-open technique, is currently the most commonly used surgical procedure for fistula management. It involves making an incision in the fistula tract, thereby opening it up, and then merging it with the anal canal, thus allowing the fistula to heal from the inside out. Intersphincteric fistulae, which do not involve the external sphincter at all, and low transsphincteric fistulae are relatively easy to treat and usually are managed by fistulotomy, with a low risk of incontinence [121]. The marsupialization of fistulotomy wounds, where the sides of the laid-open fistula tract are sutured to the skin edges of the wound, is suggested to improve healing [122]. Identification of the internal opening is the key to successful treatment with fistulotomy. If a false tract is inadvertently created, the true source of sepsis is not eradicated. Imaging studies may help guide the surgeon to the location of the internal opening. An anal retractor (usually a Hill-Ferguson) is inserted first and an attempt is made to precisely locate the internal opening by passing a probe or probes from the external to the internal opening or vice versa. Injection of hydrogen peroxide may demonstrate bubbles in the internal opening and aid in identification.
Another common procedure used to treat intersphincteric or low transsphincteric fistulae is a fistulectomy, which involves excision of the complete fistula tract. Recurrence rates are similar to those after fistulotomies; however, larger wounds are created with fistulectomies. Similar to fistulotomies, marsupialization of the fistulectomy site may result in a wound with reduced size and improved healing [122].
Setons
Because fistulotomies and fistulectomies may result in nonhealing wounds, the use of noncutting (drainage) setons is preferable. The first setons were made of horse hair but subsequent versions have consisted of silk, nylon, polyester, rubber, silicon rubber, latex, plastic, wire, and, more recently, novel materials such as self-locking cable ties. Setons are used to prevent closure of the external opening of the fistula and to allow drainage of the perianal sepsis with only minor effects on continence. There are actually few data concerning the effects on continence after the use of cutting setons; recent evidence suggests an impairment rate of about 12 %, which increases the more proximal the internal opening [123]. In this way, setons prevent abscess recurrence, although this effect is not universally successful in Crohn’s disease. Loose setons are usually used in patients with Crohn’s disease for the treatment of high perianal fistulae. Although long-term seton drainage for high Crohn’s disease perianal fistulae has been found to preserve anal sphincter function, their removal results in the recurrence of fistulae in 39 % of patients [124




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








