Fig. 37.1
Fistulous track showing the compromised muscles throughout their length. IAS internal anal sphincter, EAS external anal sphincter
Anterior Simple Anal Fistula
A simple anterior fistula is defined if the track is subcutaneous, low intersphincteric, or crosses less than 30 % of the anterior EAS. It is mandatory to identify the entire fistula track from the internal to the external opening by physical examination combined with 3D endoanal ultrasound. Anterior simple anal fistulas are safely treated by primary lay-open fistulotomy, which is preferable to fistulectomy because it involves the identification of both openings and unroofing the fistula track, without excision.
Anterior Complex Anal Fistula
Complex anterior anal fistulas must be treated using different techniques, such as setons, fibrin glue injection, a fistula plug, endorectal advancement flaps, and ligation of the intersphincteric fistula tract (LIFT).
Setons
Setons are a useful method in complex anterior anal fistula crossing less than 50 % of the anterior EAS, especially in women. A seton consists of tying an inert thread around the muscle following the fistulous tract to keep adequate drainage. It is tightened gradually according to the status of wound healing. The results are satisfactory, with minor sphincter damage and without major change in the incontinence score if comparing values before and after the operation [11], although there is some debate regarding postoperative continence in some of the limited manometric and clinical data that are available concerning the use of cutting setons [12].
Fibrin Sealant and Fistula Plug
Both methods are promising therapies that minimize incontinence risk. Initially, some authors reported success rates of 60–64 % [13, 14], but Buchanan et al. [15] conducted a prospective trial and demonstrated that only 14 % of cases remained healed after 16 months’ follow-up. Some other publications also have reported that long-term results have been disappointing [16, 17], with rates as low as 16 % [18, 19]. Concerning the fistula plug, the initial results were promising, with a success rate of 87 % [20], but the long-term results range between 29 and 48 % [17].
Endorectal Advancement Flap Closure
The use of an endorectal advancement flap is an attractive alternative for the treatment of anterior complex fistulas, especially in women, because it is aimed at avoiding the risk of severe injury to the anterior EAS, thereby preventing fecal incontinence. It shows the advantage of closing the internal opening and does not divide the sphincter; it is able to be combined with overlapping sphincter reconstruction if necessary. It involves mobilization of at least mucosa and submucosa and can include the internal anal sphincter and the rectal wall. Adequate blood supply is ensured by being sure that the base of the mobilized flap is twice the width of the apex. The flap must be advanced downward without tension, with the suture obliterating the internal opening. The external opening is left open to ensure satisfactory drainage. Successful healing rates have been reported in between 55 and 98 % of patients [21–28]. Kodner et al. [23] reported a 93 % success rate in 107 patients operated on for the treatment of low rectovaginal, anterior anoperineal, and posterior anoperineal fistulas, whereas Mizrahi et al. [27] reported a 59.6 % success rate in 94 patients, correlating poor healing with the presence of Crohn’s disease and showing a significantly higher recurrence rate (57.1 %) when compared with cryptoglandular disease fistulas (33.3 %). In addition, cancer and irradiation and a rectovaginal fistula diameter greater than 2.5 cm have been shown to be predictive factors for poor outcome [25, 29]. Although the sphincter muscles are not transected, minor incontinence has been reported in 31 % of cases and major incontinence has been reported in up to 12 % of patients [21, 23, 25, 30, 31].
Ligation of the Intersphincteric Fistula Tract (LIFT)
Originally described and published by Rojanasakul et al. [32], LIFT involves maneuvering a probe through the fistula tract and marking the skin over the intersphincteric space when the probe is in place. An incision is made in the intersphincteric groove and the fistula tract is identified in this space with the probe in situ. Once the tract is dissected free, it is encircled and the probe can be removed. Next, the fistula tract is divided and ligated. The external opening is left open and the tract is curetted. The internal opening is gently curetted as well and formally closed. The skin incision is closed with interrupted absorbable sutures. Rojanasakul et al. reported a 94 % success rate in 17 patients; however, subsequent studies of the technique have reported successful healing of the fistula in 57–89 % of patients [33–37]. More recently, Ellis [37] proposed ligation of the intersphincteric fistula tract reinforced with a bioprosthetic graft (the BioLift procedure) and reported a success rate of 94 % in 31 patients presenting with complex anal fistula.
Anal Fistulas with Crohn’s Disease
Asymptomatic Crohn’s fistulas do not require any intervention, whereas the simple low anterior fistulas may be treated by conventional fistulotomy, with rates of incontinence less than 12 % [38, 39] but a long healing time (3–6 months) [39].
Complex Crohn’s anterior anal fistulas may be treated with endorectal or anodermal advancement flaps in those patients whose rectal mucosa is grossly normal, although this is formally contraindicated in the presence of active proctitis. A technical alternative to the rectal advancement flap is the palliative long-term draining seton. The drain is tied around the fistulous track with the aim of reducing the number of subsequent septic events by providing continuous drainage and preventing closure of the external skin opening. This goal has been achieved in 48–100 % of patients, with a low rate of recurrent sepsis [38, 40, 41]. However, despite all the techniques used, the clinical course of Crohn’s disease anal fistula is completely unpredictable. Permanent remission is rare because of the recurrent nature of the disease, and between 12 and 39 % of these patients will require colostomy or a proctectomy during follow-up for progressive intestinal disease [42–44].
For further discussion of anal fistulas with Crohn’s disease, see Chap. 22.
Anovestibular Fistula
Among anorectal malformations, rectovestibular fistula is known to be the most common variety in females. AVF with a normal anus is a rare condition, affecting only 3.2 % of patients with anorectal malformations in the Western population [45]; however, higher incidences have been reported in Asian countries [46, 47]. This malformation has been labeled variously as an N-type fistula, an H-type anorectal fistula, a common perineal canal, and a double end of the alimentary tract [45–48]. Associated anomalies are less common in females, but in males these malformations often are combined with severe additional renal, esophageal, and spinal abnormalities. Vestibular fistula is the commonest anorectal malformation in female children and constitutes 10–30 % of large reported series [45]. The bowel opens between the vagina and the fourchette. Most reports categorize vestibular fistula as a low anomaly; however, Heinen [49] has considered such a malformation as an intermediate anomaly. There are differences between rectovestibular fistula and AVF: the former is an intermediate anomaly (according to the Wingspread classification) because the rectum ends above the top end of the striated muscle complex with a longer fistula opening at the vestibule. AVF is a low anomaly, where the bowel traverses the striated muscle complex [50, 51].
More importantly, the gravity of its surgical correction is frequently underestimated; in addition, a significantly high incidence of postoperative constipation when compared with other anorectal malformations makes AVF a unique anomaly. In comparison with male anomalies or other more complex high female anomalies, it has received less attention than it probably deserves. The diagnosis is made by clinical examination and by a suitable Hegar dilator inserted into the fistula. The sacrum is examined in all patients and if any abnormality is found, a lateral spine x-ray should be performed. Ultrasound of the abdomen and echocardiography also are required to identify any intra-abdominal (renal) and cardiac anomaly.
During the neonatal period, a cut-back procedure is recommended for AVF, and thereafter, various methods are available for its definitive correction. These include a Y-V plasty [45], perineal anal transposition (Potts) [46], X plasty [47], and X-Z plasty [48]. Inadequate exposure, incomplete rectovaginal separation with a subsequent tendency for the neoanus to move forward, and blind placement of the anorectum in the sphincter complex are the main disadvantages of these techniques, making them less popular. Better exposure and precise placement of the anal canal within the external sphincter complex have made the posterior and anterior sagittal approaches more popular and established for the correction of AVF [46]. There are only limited data concerning the continence status of adults patients after these neonatal procedures and their need for revision [52–54].
There is still controversy regarding the performance of a protective colostomy in AVF, although this is considered the safest option for these cases [55, 56]. Fistula repair with a protective colostomy is preferred by some surgeons to reduce the risk of wound breakdown [46, 48]. Recurrences are not uncommon and they still have occurred even after a preliminary colostomy [46, 48, 56]. Banu et al. [57] have recommended primary surgery without a temporary stoma and they have reported wound disruption only in patients with preoperative perineal abscesses. Another option for these patients would be to treat the abscess initially and then to perform the definitive procedure as a second stage.
Anterior Sagittal Anorectoplasty
Anterior sagittal anorectoplasty has been reported in some centers in India and Asia as well as in the United States, South America, and Europe as both a primary [58–61] and a secondary procedure [62]. Preliminary on-table cutaneous electromyography is advisable for the determination of the correct positioning of the neoanus [63]. A racket-shaped incision is performed around the neoanus and extended toward the vagina, following the fistula tract (Fig. 37.2a). The rectum is separated from the vagina by a combination of blunt and sharp dissection, taking care to preserve the integrity of the rectal wall (Fig. 37.2b). There are often dense adhesions of the rectum to the vagina at the fistula site; however, once this is crossed, rectovaginal separation is comparatively easy in both anovestibular and the rectovestibular anomalies (Fig. 37.2c). On occasion, damage may occur to the vaginal wall during separation, although this is easily sutured when the rectal mobilization is complete. The end of the dissection is indicated when the rectum lies without tension 5 mm beyond the skin of the perineum (Fig. 37.2d). The striated muscle complex is delineated by electrostimulation and the rectum anchored to this active area of the sphincter. The perineal body is reconstructed by suturing the tissue between the rectum and vagina in layers (Fig. 37.2e, f). Finally, the fourchette is reconstructed, an anoplasty is performed, and the incision is closed primarily (Fig. 37.2g).
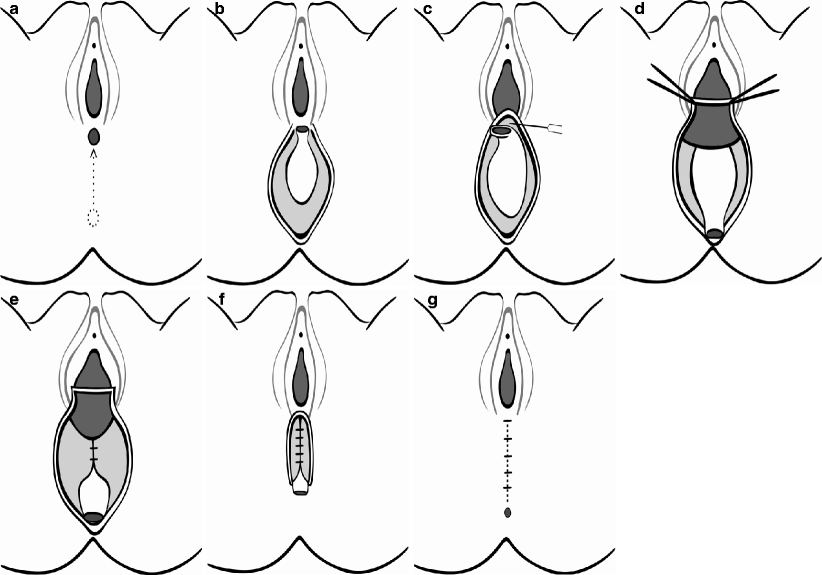

Fig. 37.2
(a) A midline incision extending from the fistula to the center of the proposed new anus. The incision is deepened by dividing the perineal muscles and the anterior fibers of the external anal sphincter complex. (b) The posterior rectal wall is exposed. There is a well-defined plane posterior to the rectum where blunt dissection can be performed. (c) Sharp dissection is required to enter the correct plane between the posterior vaginal wall and the rectum. (d) The rectal mobilization is completed. The deeper anterior dissection is performed simultaneously with the remaining posterior and lateral dissections. During the posterior dissection, the levator ani muscle does not require division in the case of a vestibular anus. (e) Perineal reconstruction is commenced. The mobilized anorectum is placed within the limits of the external anal sphincter complex. The sutures initially are placed in the deepest part of the soft tissues. (f) The perineum is completely reconstructed. A few sutures are employed circumferentially to fix the lateral and posterior walls of the rectum to the surrounding muscle. (g) The completed prcedure after skin closure
Since the inception of anterior sagittal anorectoplasty, the basic management of vestibular fistula has remained unchanged; however, the rate of complications has decreased by 7 % overall [60]. The factors responsible for the improvement of results include better surgical technique and dissection with growing experience, less tissue trauma, adequate rectal mobilization, and an absence of hemorrhage leading to hematoma [60, 61]. The availability of lactulose to keep the stool soft during the immediate postoperative period is also a factor responsible for the improved results. It is advisable that these patients undergo cesarean delivery in the future in an effort to prevent damage to the perineal body incurred during vaginal delivery [59].









