Solid-organ transplant recipients are at increased risk for the development of skin cancers. A promising strategy for managing these complex patients involves a multidisciplinary approach that incorporates clinicians from various specialties, which provides the optimal milieu for patient education, treatment, and follow-up. The multidisciplinary clinic also facilitates communication between dermatologists and transplant physicians regarding such crucial concerns as revision of immunosuppression. This article reviews the problem of skin cancer in solid-organ transplant recipients, outlines preventive measures, discusses therapeutic modalities, and reinforces the advantage of a multidisciplinary approach in the management of this population.
There are presently more than 170,000 solid-organ transplant recipients (SOTR) living in the United States alone, compared with 120,000 SOTR 10 years ago. This increase is attributable to an increase in the number of transplants performed and to improved posttransplant survival as a result of refinement of surgical techniques and medical management of the SOTR. As transplant medicine continues to advance, posttransplant survival may be expected to improve further and the numbers to increase accordingly. These trends are of particular interest to the dermatologist because SOTR are known to be at markedly increased risk for cutaneous malignancies. More than 50% of SOTR are ultimately diagnosed with at least 1 skin cancer. Skin cancers remain the most commonly diagnosed neoplasms among SOTR, comprising nearly 40% of all posttransplant malignancies. In addition to the increased incidence of these tumors in the SOTR population, the tumors tend to behave more aggressively than those in nontransplant hosts.
The significant burden of morbidity and mortality conferred by these aggressive skin cancers demands a systematic response by dermatologists caring for the SOTR population. In the past, these patients were typically not brought to the attention of dermatologists until after they were discovered to have cutaneous malignances, obviating the possibility of implementing effective preventive strategies. This suboptimal approach to a population already known to be at higher risk for skin cancer has been characterized as reactive rather than proactive. In recent years, an alternative approach has been developed at several centers: the organization of a dedicated dermatology subspecialty clinic within a multidisciplinary clinical environment in the transplant center. Such a multidisciplinary clinic may integrate transplant surgeons, dermatologists (including dermatopathologists and Mohs micrographic surgeons), nephrologists, hepatologists, medical and radiation oncologists, and representatives of other relevant subspecialties. This multidisciplinary environment facilitates collaboration and close communication between the wide array of specialties responsible for the various facets of the care of a complex patient population. In addition, it serves to simplify the expeditious scheduling of follow-up with the necessary specialists, such that the dermatologist may routinely see the SOTR in the pretransplant phase to undertake baseline risk assessment and initiation of appropriate interventions. Fundamentally, the role of the dermatologist in this multidisciplinary approach follows the paradigm of prevention (via education, chemoprophylaxis, and revision of immunosuppression), surveillance for the purpose of early detection, and treatment of cutaneous malignancies.
Epidemiology
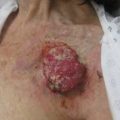
Most the cutaneous malignancies diagnosed in SOTR are nonmelanoma skin cancers (NMSC), with squamous cell carcinomas (SCC) and basal cell carcinomas (BCC) accounting for 90% to 95% of the total in multiple reported cohorts. The mean interval between transplantation and diagnosis of NMSC varies with patient age at transplantation: 8 years for patients transplanted around 40 years of age, but approximately 3 years for those transplanted after 60 years of age. Although the incidence of both tumor types is markedly increased in SOTR, the rate of SCC is disproportionately higher ( Table 1 ). Although the incidence of BCC is increased tenfold to 16-fold, SCC occurs at a frequency of between 65 and 250 times that of the general population. This results in the inversion of the SCC/BCC ratio of 1:4 in the general population to a ratio of at least 4:1 in SOTR. This reversal becomes even more pronounced with decreasing latitude (ie, in sunnier climates) and length of time after transplant.
| Skin Cancer | Fold Increase in Incidence |
|---|---|
| Squamous cell carcinoma | 65–250 |
| Basal cell carcinoma | 10–16 |
| Melanoma | 2.2–8 |
| Kaposi sarcoma | 84 |
In an Australian cohort, the cumulative incidence of NMSC has been reported to be 45% by ∼10 years after transplantation, and 70% by 20 years after transplantation. In a UK cohort, the mean annual risk of developing NMSC was found to be 3.27% for SOTR less than 5 years after transplantation, 5.86% for SOTR 5 to 10 years after transplantation, and 11.1% for those more than 10 years after transplantation. The considerable acceleration of SCC incidence in SOTR is such that the diagnosis of a first SCC has been shown to be predictive of multiple subsequent NMSC within 5 years. In addition to the increased incidence of SCC, the tumors display a more aggressive phenotype in SOTR than in the general population, with more rapid growth, local recurrence in 13.4% of SOTR, and a metastatic rate of approximately 8%.
The risk of other types of skin cancer is also increased in the SOTR population (see Table 1 ). The incidence of melanoma seems to be increased 2.2-fold to 8-fold in SOTR. The largest study to date of melanoma in SOTR found that risk of melanoma increased with increasing age in male patients, but not in female patients. The incidence of Kaposi sarcoma has been shown to be increased 84-fold in SOTR. SOTR may be at increased risk for other rare skin cancers, including Merkel cell carcinoma, atypical fibroxanthoma, angiosarcoma, verrucous carcinoma, leiomyosarcoma, and cutaneous T-cell and B-cell lymphomas, but evidence is scant and anecdotal at present owing to the rarity of these tumors.
Risk factors
The determinants of skin cancer development in SOTR are well defined ( Box 1 ). As in the general population, the most significant risk factor in SOTR is exposure to ultraviolet radiation (UVR). Fair skin (Fitzpatrick skin types I, II, or III) also predisposes SOTR to cutaneous malignancies. Increased age is correlated with the development of skin cancer, probably because of greater total UVR exposure in older patients.
Exposure to UVR
Fitzpatrick skin types I, II, or III
Increased age at transplantation
Duration, degree, and type of immunosuppression
Type of organ transplant: heart/lung > kidney > liver
Previous organ transplant
Personal history of actinic keratosis (AK), NMSC, or melanoma
Human papillomavirus (HPV) infection
Several additional risk factors are unique to the SOTR population. The most important of these is the immunosuppression that accompanies organ transplantation. Pharmacologic suppression of the immune system increases the risk of developing malignancies, particularly those of the skin. Moreover, it has been shown that their incidence is proportional to the dosage, duration, and even the type of immunosuppression. Among SOTR, heart transplant recipients seem to be at the highest risk for developing skin cancers after transplantation (2–3 times that of kidney transplant recipients), because of the greater intensity of immunosuppression demanded by the cardiac allograft. Kidney transplant recipients tend to develop more individual tumors in the course of time, possibly as a result of their younger average age at transplantation and consequent longer ultimate duration of immunosuppression. Liver transplant recipients seem to be at the lowest relative risk for skin cancers. Repeat or multiple transplantation seems to further increase skin cancer risk, likely as a result of the greater cumulative burden of immunosuppression.
Although human papillomavirus (HPV) is a known oncovirus (implicated in the carcinogenesis of anogenital malignancies), its role in the pathogenesis of NMSC has been uncertain. However, recent data seem to confirm that HPV does contribute to the development of SCC (especially in the immunocompromised host), although the precise mechanism remains a matter of debate. Several genetic polymorphisms, including those in glutathione S -transferase, interleukin 10, the folate pathway, and vitamin D receptor genes, may also contribute to skin cancer development in kidney transplant recipients in particular, although it remains to be elucidated whether this applies to SOTR in general. Another important risk factor among SOTR is pretransplant personal history of AK, NMSC, or melanoma, which significantly increases the risk of subsequent skin cancer development in the posttransplant period.
Such factors as sex of the recipient, type of donor (live vs cadaveric), and duration of pretransplant dialysis (in kidney transplant recipients) do not seem to increase the risk of cutaneous malignancies in SOTR.
Risk factors
The determinants of skin cancer development in SOTR are well defined ( Box 1 ). As in the general population, the most significant risk factor in SOTR is exposure to ultraviolet radiation (UVR). Fair skin (Fitzpatrick skin types I, II, or III) also predisposes SOTR to cutaneous malignancies. Increased age is correlated with the development of skin cancer, probably because of greater total UVR exposure in older patients.
Exposure to UVR
Fitzpatrick skin types I, II, or III
Increased age at transplantation
Duration, degree, and type of immunosuppression
Type of organ transplant: heart/lung > kidney > liver
Previous organ transplant
Personal history of actinic keratosis (AK), NMSC, or melanoma
Human papillomavirus (HPV) infection
Several additional risk factors are unique to the SOTR population. The most important of these is the immunosuppression that accompanies organ transplantation. Pharmacologic suppression of the immune system increases the risk of developing malignancies, particularly those of the skin. Moreover, it has been shown that their incidence is proportional to the dosage, duration, and even the type of immunosuppression. Among SOTR, heart transplant recipients seem to be at the highest risk for developing skin cancers after transplantation (2–3 times that of kidney transplant recipients), because of the greater intensity of immunosuppression demanded by the cardiac allograft. Kidney transplant recipients tend to develop more individual tumors in the course of time, possibly as a result of their younger average age at transplantation and consequent longer ultimate duration of immunosuppression. Liver transplant recipients seem to be at the lowest relative risk for skin cancers. Repeat or multiple transplantation seems to further increase skin cancer risk, likely as a result of the greater cumulative burden of immunosuppression.
Although human papillomavirus (HPV) is a known oncovirus (implicated in the carcinogenesis of anogenital malignancies), its role in the pathogenesis of NMSC has been uncertain. However, recent data seem to confirm that HPV does contribute to the development of SCC (especially in the immunocompromised host), although the precise mechanism remains a matter of debate. Several genetic polymorphisms, including those in glutathione S -transferase, interleukin 10, the folate pathway, and vitamin D receptor genes, may also contribute to skin cancer development in kidney transplant recipients in particular, although it remains to be elucidated whether this applies to SOTR in general. Another important risk factor among SOTR is pretransplant personal history of AK, NMSC, or melanoma, which significantly increases the risk of subsequent skin cancer development in the posttransplant period.
Such factors as sex of the recipient, type of donor (live vs cadaveric), and duration of pretransplant dialysis (in kidney transplant recipients) do not seem to increase the risk of cutaneous malignancies in SOTR.
Pretransplant considerations
In addition to management of the posttransplant patient, the dermatologist may serve a significant advisory role in the pretransplant phase. As noted earlier, a personal history of skin cancer (or premalignant skin lesions) is known to be an independent risk factor for development of further skin cancers after transplantation. There has therefore been some discussion in the recent literature as to whether a previous diagnosis of high-risk skin cancer should, a priori, be considered a relative contraindication to organ transplantation.
A guiding principle of the organ transplantation process is the judicious allocation of a scarce resource to those likely to derive the most benefit. Accordingly, as with other malignancies, patients with active metastatic skin cancer would not qualify as transplant candidates. However, the question remains whether patients with a history of intermediate-risk to high-risk tumors should be transplanted, given that the aggressiveness and metastatic potential of their malignancy may even be increased in the posttransplant setting. Little evidence yet exists to guide transplant physicians in answering this question. Therefore, a multidisciplinary approach is warranted. The decision must be based on careful examination of the historical and pathologic data of the cutaneous malignancy in the potential transplant candidate, in consultation with appropriate specialists, including dermatopathologists and dermatologic surgeons. Otley and colleagues proposed suggested waiting intervals for reevaluation before transplantation after various cutaneous malignancies. These proposals have been cited with approval by other investigators, and may form the basis of a standard approach to pretransplant evaluation of such patients in the multidisciplinary setting.
Preventive education
Because the most important element of skin cancer prevention in SOTR is minimization of exposure to UVR, education regarding sun protection and avoidance is the cornerstone of any effective prevention program. However, numerous studies have shown both knowledge of the importance of photoprotection and compliance with photoprotective measures to be consistently inadequate among SOTR. Before the advent of multidisciplinary transplant clinics, Seukeran and colleagues proposed that these deficiencies may be attributable, at least in part, to insufficient input by dermatologists in the process of preventive education.
It is thus incumbent on the dermatologist to provide rigorous, repetitive, and persuasive instruction to patients regarding the necessity of photoprotection and the most efficacious means thereof. Several studies have shown that the multidisciplinary approach is particularly well suited to the accomplishment of this goal. Ismail and colleagues reported that, among patients attending their specialist organ transplant recipient dermatology clinic, 98% recalled receiving photoprotection advice and 95% reported regular sunscreen use, compared with 77% and 67%, respectively, of patients who did not attend. Clowers-Webb and colleagues described significantly more compliant sun-protective behavior among SOTR in a multidisciplinary setting who received intensive versus standard preventive education. Ulrich and colleagues found that, regardless of general photoprotection education, provision of free sunscreen and specific training regarding its correct application resulted in significantly more photoprotection compliance (measured as sunscreen use 5.6 d/wk, compared with 0.3 d/wk in the control group), with a concomitant decline in NMSC incidence. The dermatologist in the multidisciplinary setting should frequently and repeatedly encourage all SOTR to apply sunscreen (of sufficient sun protection factor) every day, not just when sun exposure is expected, and counsel avoidance of sun exposure between 10:00 am and 2:00 pm , abstention from exposure to artificial sources of UVR (ie, tanning beds), and dressing in sun-impermeable clothes including long-sleeved shirts, long pants, and broad-brimmed hats. Patients practicing optimal photoprotection may be at risk for vitamin D deficiency, because of reduction of vitamin D production in non–sun-exposed skin. Therefore, the American Academy of Dermatology recommends oral supplementation of these patients with a total daily dose of 1000 IU of vitamin D to prevent deficiency while minimizing skin cancer risk.
Revision of immunosuppression
Although pharmacologic suppression of the host immune system is indispensable to graft survival in SOTR, an unintended consequence of chronic immunosuppression is acceleration of the development of cutaneous malignancies. Immunosuppressant medications promote tumorigenesis by 2 mechanisms. First, the agents may be directly carcinogenic. Second, the immunosuppressive milieu impairs endogenous detection and destruction of cells harboring mutations, which may progress to malignancy if left unchecked. Reducing the degree of immunosuppression may be a viable adjuvant strategy for retarding tumor development in select cases. Conceptually, the usefulness of such an approach is contingent on weighing the benefit conferred by reducing skin cancer incidence against the risk of precipitating graft rejection.
To date, only 1 randomized controlled trial (RCT) has evaluated reduction of immunosuppression. Dantal and colleagues found that reduction of immunosuppression did not result in significant impairment of graft survival (compared with a standard-dose control group), whereas it did decrease the incidence of new skin cancers during the duration of follow-up. Based on observational data, reduction of immunosuppression may also serve to mitigate the aggressiveness, as well as the multiplicity, of high-risk or metastatic NMSC. Although no guidelines yet exist regarding reduction of immunosuppression, the International Transplant Skin Cancer Collaborative (ITSCC) and Skin Care in Organ Transplant Patients Europe (SCOPE) have published an expert consensus survey of transplant dermatologists recommending this strategy for secondary prevention in high-risk cases of NMSC and melanoma. A follow-up study surveyed nondermatologist transplant physicians and found them to be even more amenable to aggressive reduction of immunosuppression in similar clinical circumstances. Both these studies emphasize that decisions regarding reduction of immunosuppression should be undertaken via a multidisciplinary approach with appropriate input from both dermatologists and transplant physicians.
Another method of revising immunosuppression in SOTR entails altering the type of immunosuppressant regimen itself. In the past, this was commonly accomplished by maintaining SOTR on fewer total immunosuppressant agents, which seems to be efficacious in reducing the incidence of skin cancers. In recent years, evidence has begun to accumulate that mammalian target of rapamycin (mTOR) inhibitors, such as sirolimus and everolimus, may confer a decreased risk of skin cancer relative to traditional calcineurin inhibitors. Moreover, data suggest that mTOR inhibitors may exert a protective effect on the development of skin cancers via their incidental antineoplastic activity. Several RCT are ongoing to prospectively evaluate the effect of mTOR inhibitors (compared with calcineurin inhibitors) on skin cancer risk in SOTR, which will help to elucidate the future role of these promising agents. Studies in the transplant literature have already shown the safety and efficacy of mTOR inhibitors in graft function and survival. The mTOR inhibitors have their own side effects (including hyperlipidemia, myelosuppression, impaired wound healing, proteinuria, and pneumonitis) and there is insufficient evidence to recommend their use as first-line agents in de novo SOTR. Any decision regarding conversion of immunosuppression must be made on a case-by-case basis involving close consultation between the dermatologist and the primary transplant team.
Chemoprophylaxis
The tendency of skin cancers to arise from premalignant keratotic lesions on sun-damaged skin in SOTR suggests field cancerization. The concept of field cancerization denotes a process whereby an area subject to an ongoing carcinogenic insult may accumulate repeated mutations until 1 focus of that area undergoes malignant transformation and clonal expansion, forming a clinically apparent tumor. Although the malignant neoplasm may arise from a single offending cell, a concentric field of surrounding tissue still harbors an analogous complement of mutations and consequent malignant potential, which standard excisional or ablative techniques for skin cancer fail to eliminate. Thus, modalities for the suppression of these reservoirs of malignant precursors are indicated in SOTR at high risk for future cancer development.
Retinoid derivatives have been shown to be the most efficacious agents available for this purpose. The usefulness of systemic retinoids for suppression of SCC development has been widely investigated, although study designs vary. Two RCT have shown a significant decrease in the incidence of both AK and SCC with administration of oral acitretin. A third RCT noted a reduction in AK but did not show a significant decrease in the incidence of SCC. Two other studies also found a decrease in the incidence of both AK and SCC with acitretin, although both were small and lacked formal control groups. A retrospective longitudinal study published recently confirmed the long-term efficacy of acitretin for suppression of SCC. Accordingly, the available evidence supports the adjuvant use of retinoid chemoprophylaxis in select SOTR at high risk for developing multiple, recurrent, and aggressive NMSC. Indications for the initiation of systemic retinoid therapy are presented in Box 2 .
Development of multiple SCC per year (5–10/y)
Development of multiple SCC in high-risk locations (eg, head and neck)
SOTR with SCC and a history of lymphoma/leukemia
Single SCC with high metastatic risk
Metastatic SCC
Explosive SCC development
Eruptive keratoacanthomas
Poor tolerability adversely affects compliance with systemic retinoids. Patients commonly complain of mucocutaneous xerosis and arthralgias/myalgias, frequently leading to self-discontinuation of the drug. Because these effects are generally dose dependent, acitretin should be started at a low dose (10 mg/d) and increased incrementally at 2-week to 4-week intervals to target dose of 20 to 25 mg/d, enabling patients to acclimate gradually to any adverse effects. Laboratory abnormalities such as increased transaminases and hyperlipidemia are also common, requiring regular monitoring. In the interest of minimizing systemic side effects, topical retinoid formulations have also been studied, but with inconsistent results.
Notable among all the retinoid studies mentioned earlier is the consistent observation of a rebound effect: relapse of SCC on discontinuation of the drug. Thus, barring severe adverse effects necessitating discontinuation, maintenance of retinoid chemoprophylaxis should be lifelong. To promote compliance, patients should be counseled extensively regarding expectation and manageability of side effects, and the importance of continuing retinoid therapy once initiated. Patients should be encouraged to discuss their concerns with their transplant dermatologist so that strategies for mitigating intolerable side effects (such as dose reduction) may be implemented without sacrificing chemosuppression.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree