INTRODUCTION TO SCAR FORMATION AND WOUND HEALING
Civilians and wounded warriors all over the world struggle with functional and symptomatic issues due to hypertrophic and keloid scars. Scars belong to all medical specialties; however, as skin experts, dermatologists have been the leading pioneers in the management and treatment of these conditions. Through the development of therapeutic modalities, including lasers, the specialty of dermatology has propelled the field of scar therapeutics forward.
This chapter will focus on hypertrophic and keloid scarring. It will review how these entities arise as complications from the phases of wound healing as well as the genetic and environmental factors that contribute to their formation. Finally, we will review treatment strategies and emerging treatment modalities for these conditions.
Scarring must be understood where it begins—at the cellular and molecular level. Wound healing is a multistep process with overlapping phases. The phases are classified as inflammatory, proliferative, and remodeling. The inflammatory phase starts immediately after injury and completes within the first 48 to 72 hours. It begins with activation of the clotting cascade resulting in release of cytokines IL-6 and IL-8 and several chemokines. Immune cells arrive to the site of injury and local cells proliferate. Release of TGF-β results in fibroblast recruitment, thus starting the proliferative phase. This phase can last up to 7 weeks following injury. Granulation tissue begins to form and is made up of macrophages, fibroblasts, proteoglycans, hyaluronic acid, elastin, and procollagen. Ongoing signaling by TGF-β, released by macrophages, activates fibroblasts and induces collagen III formation. Myofibroblasts start the process of wound contraction. The proliferative phase also initiates angiogenesis, and the wound begins to reepithelialize as keratinocytes from the wound periphery migrate across the wound surface. The remodeling phase is characterized by wound maturation and completes in 1 year. Normal extracellular matrix remodeling of the scar takes several months. Initially the extracellular matrix is composed of fibrin and fibronectin. About 2 to 3 weeks after injury, stress on the wound stimulates deposition of collagen type III. Matrix metalloproteinase deposition is a rate-limiting step in collagen remodeling. There is a delicate balance between destruction of immature and formation of mature wound structures. Over time, collagen type III is replaced by collagen type I, forming stronger scar tissue. This type I collagen is arranged in bundles parallel to the epidermis, in nonpathologic scar formation. Immature blood vessels formed during proliferation regress. Fibroblasts also play an important role in scarring. The deeper reticular fibroblasts have a specific phenotype and produce more TGF-β3, connective tissue growth factor, and heat shock protein 47. Scarring only occurs after a threshold of dermal injury and activation of fibrotic fibroblasts. Langerhans cells are found in greater numbers in hypertrophic scars and recruit T cells. Keratinocytes of hypertrophic scars have a higher proliferative index with increased Ki67, K5/14 expression, and aberrant expression of hyperproliferative keratin K6 and K16.
Another major force in scar formation is mechanotransduction. This perpetuates both hypertrophic scars and keloids from underlying tension. There are over 1000 genes regulated by wound tension. Tensegrity is the alignment of structural components of the cytoskeleton in response to mechanical forces to preserve the cell’s tensional integrity. During inflammation these forces interact and upregulate cell signaling pathways and gene expression leading to scarring.
Differences Between Hypertrophic Scars and Keloids
Both hypertrophic and keloid scars are characterized by imbalanced wound healing processes. Both lesions have some component of fibroblast overproduction indicating either excess healing signals or failure of their downregulation. Histology of hypertrophic scars and keloids reveals increased vasculature, increased inflammation, and dense fibroblasts.
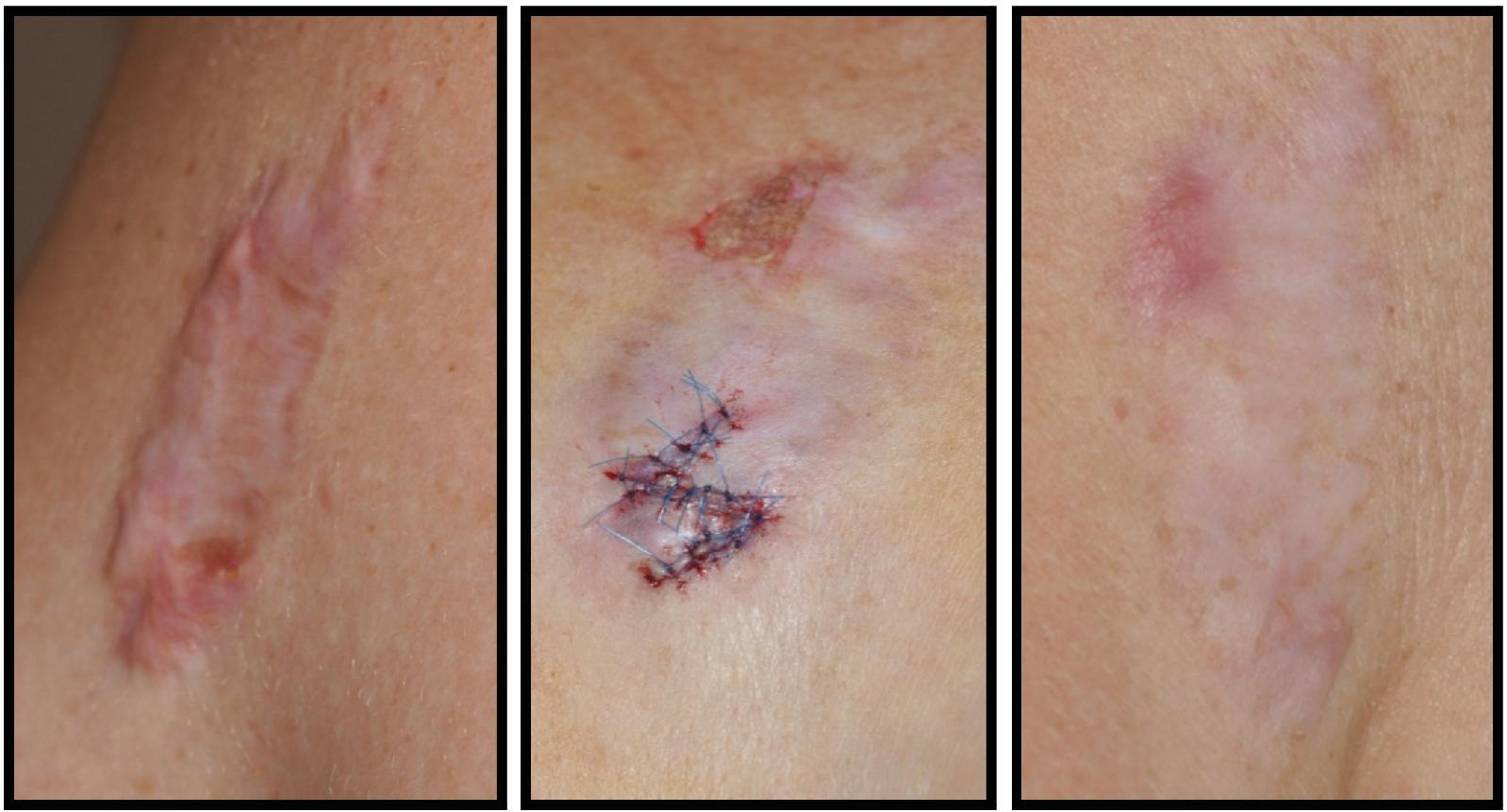
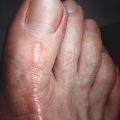
Hypertrophic scars are clinically distinguished as firm, raised scar tissue contained within the boundary of the original wound ( Fig. 8.1 ). They typically arise between 4 and 8 weeks following the injury. These scars tend to occur at areas of high tension, such as the chest and shoulders. Histologically, they maintain an organized parallel pattern of collagen and are made up primarily of type III collagen. There are abundant nodules with myofibroblasts that form during the healing process when the wound is under high tension. Hypertrophic scars can regress over time, but it may take years to see this effect.
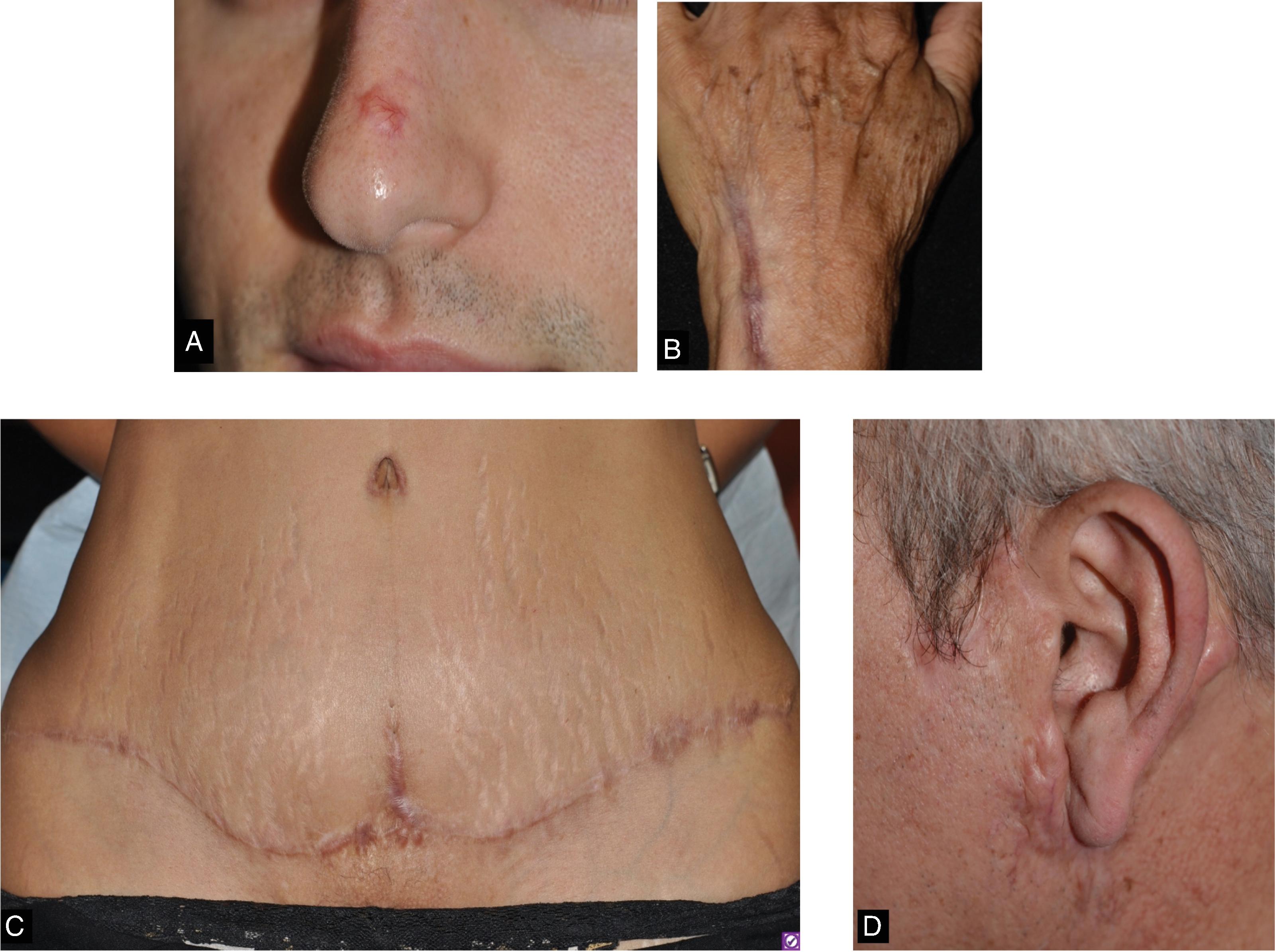
Keloid scars may appear at variable time points following the initial injury. Clinically they are distinguished as firm scar tissue extending beyond the boundary of the original wound ( Fig. 8.2 ). They exhibit ongoing evolution without regression. Histologically, keloid scars are distinguished by their random and haphazard collagen organization. They contain both collagen type I and collagen type III. The keloidal scar tissue infiltrates surrounding normal tissue.
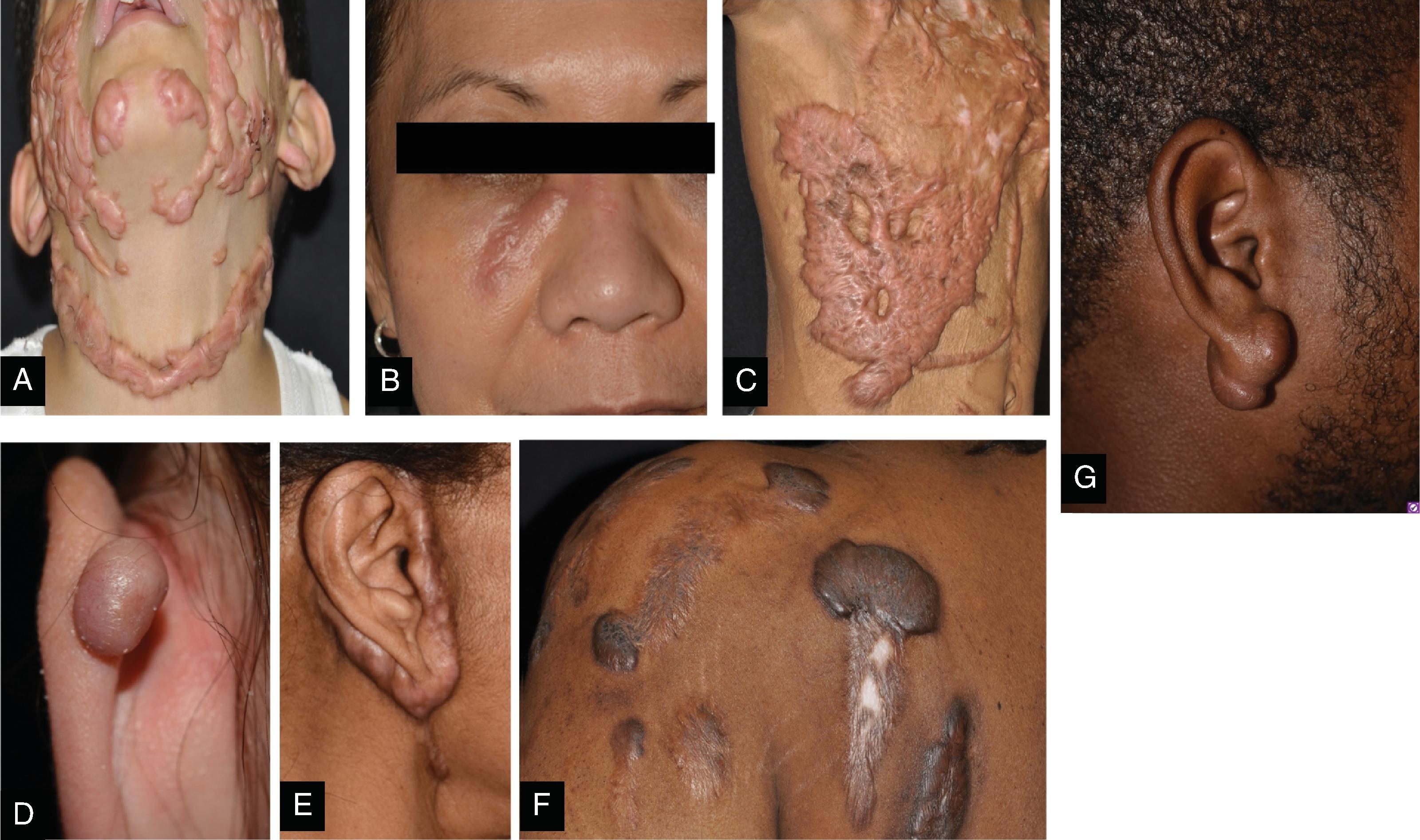
Patient Populations and Characteristics
Keloid formation follows a bimodal age distribution but is more common in younger age groups, between the first and third decades of life. It is interesting to note that humans are the only animals that form keloids. Keloids are thought to be derived from abnormal fibroblasts. They contain increased growth factor receptors and therefore exhibit more robust responses to TFG-β signaling among other growth factors. There is loss of downregulation during the collagen and extracellular matrix production resulting in decreased synthesis of products that promote matrix breakdown and collagen organization. A higher prevalence is noted in populations of African and Asian descent, especially those of darker skin types. Local factors that increase an individual’s risk of forming aberrant scars include wounds under high tension, endocrine dysfunction, tissue hypoxia, and autoimmune conditions. Large population-based surveys have revealed that a family history of keloid scars increases an individual’s odds of forming keloids themselves. Inheritance patterns can be autosomal dominant or autosomal recessive, and there appears to be variable penetrance and expression of relevant genes. Although much of the exact molecular mechanisms are still under investigation, there is a clear interplay between environmental factors, genetic predisposition, medical comorbidities, and the wound-healing process.
Genes Associated With Keloid Formation
The three isoforms of TGF-β are the primary drivers of pathologic scar formation. Fetal skin, which does not scar, has been shown to express a higher ratio of TGF-β3 to the β1 and β2 isoforms. Conversely, this ratio is decreased in adult skin. Interestingly, the oral mucosa that is known for rapid healing with minimal scarring has an elevated TGF-β3 to β1 and β2 ratio. A number of in vitro studies have affirmed that TGF-β3 prevents scarring while expression of TGF-β2 and TGF-β1 contribute to scarring.
Scar Assessments—Clinical and Research Tools and Scales
There are many invasive and noninvasive scar assessment tools; however, there is no current consensus for a single valid and reliable noninvasive assessment instrument for the quantification of the characteristics of all cutaneous scars. The exact requirements of an objective tool should be considered before deciding on which scar assessments are chosen. Consideration of the parameter(s) to be measured, symptom(s) of patients, and goal(s) of the clinical trial is essential for objective evaluation of skin scarring. Table 8.1 shows different objective scar tools that may be utilized.
| Physical Measures of Elasticity | Physical Measures of Collagen | Physical Measures of Color |
|---|---|---|
| Elasticity Optical topography Ultrasound Extensometer SkinFibroMeter Skin Scanner PRIMOS pico * Chromameter Cutometer Elastometer | Ultrasound—thickness, scar density/signal intensity Optical coherence tomography—collagen intensity Reflectance confocal microscopy Torque meter—elastic deformation, elastic recovery, total extensibility | Tristimulus color systems—Chromameter Narrow-band reflectance spectrophotometer Planimetry 3D imaging Laser Doppler flowmetry Capillaroscopy |
* PRIMOS pico is a a three-dimensional, highresolution, non-invasive clinical imaging device.
A number of scales exist for standardized assessment of scars. Characteristics used to evaluate scars include firmness, pigmentation, height, surface area, texture, and vasculature. The Vancouver Scar Scale scores vascularity, thickness, and pigmentation. This scale has been used in many studies analyzing scar outcomes in burn patients, but it lacks patient subjectivity and symptoms. The Patient and Observer Scar Assessment Scale adds surface area of the scar and patient appraisals of symptoms such as pruritus, pain, color, stiffness, thickness, and relief. Other scales in practice include the Visual Analogue Scale with scar ranking, the Manchester Scar Scale, and the Stony Brook Scar Evaluation Scale, among others. All scales consider different variations of cosmesis from either the patient and/or the clinician’s perspective. All have their own downfalls and disadvantages. However, the basis of their use lies in determining the aspects of the scar that impact the patient and can serve as guides when selecting treatment targets in their revision.
One problem with the Vancouver Scar Scale is that it was developed in Vancouver and the population of scar patients was 85.3% European with fair skin types. In our diverse world we need scar scales that reflect all skin types. Lyons et al., 2023 from Henry Ford in Detroit, Michigan, studied disease severity and quality-of-life outcomes measurements in patients with keloids via a systemic review. They realized a new scale was needed to standardize keloid assessments for diverse skin population and patient-reported symptomatology. This led to the development of the Detroit Keloid Scale to establish a new keloid scale to bridge this gap. With seven questions, the Detroit Keloid Scale is easy to administer and has shown observer interrater reliability as well as total scale reliability.
PREVENTIVE TREATMENTS AND NONPROCEDURAL INTERVENTIONS
Treating scars is a multidisciplinary endeavor. Many professionals including acute trauma and burn surgeons, general surgeons, reconstructive surgeons, dermatologists, psychiatrists, physical therapists, occupational therapists, and many more may be needed to heal scar patients over the course of years. In addition to surgery and lasers, there are also other technologies available to help with scar improvement, including massage, occlusive dressings, radiation, and others.
Wound Care, Dressings, and Topical Therapies for Scar Prevention
There are a number of preventive mechanisms to reduce the risk of aberrant scar formation. They begin with minimizing the stimuli known to promote scar proliferation. It is paramount that closures are achieved with little to no tension. It is also important to minimize postoperative complications (particularly infection) by adequately cleansing, debriding, and achieving hemostasis. Patients should be counseled to start using sunscreen as early as 10 days postsurgery (once completely reepithelialized) to prevent scar discoloration.
Occlusive dressings should be used as first-line agents for keloid prevention. Broadly speaking, they are thought to help with wound hydration that influences growth factor secretion and fibroblast regulation. Silicone-based dressings have been shown to improve scar erythema, pliability, and thickness. They may be used during early wound healing through scar maturation. However, the therapeutic effect is not necessarily unique to silicone therapies as a systematic review of 20 trials with 870 patients concluded that many studies demonstrating silicone efficacy compared to nonsilicone dressings were highly biased and of poor quality. Other types of occlusive dressings include non–silicone-containing sheets; adhesives; and microporous, polyurethane, and hypoallergenic tapes. Use of these dressings requires consistent application for the majority of the day for up to 12 months to see positive effects. It has been reported that pressure combined with silicone gel sheets provided excellent improvement in 23% of patients, moderate improvement in 37.5%, and no or slight improvement in 28%.
Steroid tapes/plasters have long been used in Japan as first-line therapy for keloids and hypertrophic scars. They function to soften and thin out scars and may be used in combination with intralesional steroid injections. They should be applied daily for the majority of the day, if not a full 24 hours, for several weeks to months to see results. One protocol involves using the plaster therapy 1 month following surgery for 24 hours per day to suppress aberrant scar recurrence for three at least 3 months. Another protocol involves daily application of steroid tape (4 g/cm 2 flurandrenolone) over 6 to 8 weeks. Consistent use has been reported to improve scar elevation, erythema, and pruritus after 12 months. The most common side effect of this modality is contact dermatitis, which may be mitigated by decreasing use time and or increasing the frequency with which it is replaced.
Scar massage . Physical medicine and rehabilitation medicine has taught us that in addition to our medical therapies, massage has a role in treating hypertrophic scars and keloids. Scar massage helps decrease scar tightness, increase skin mobility, and increase range of motion when combined with other medical therapies.
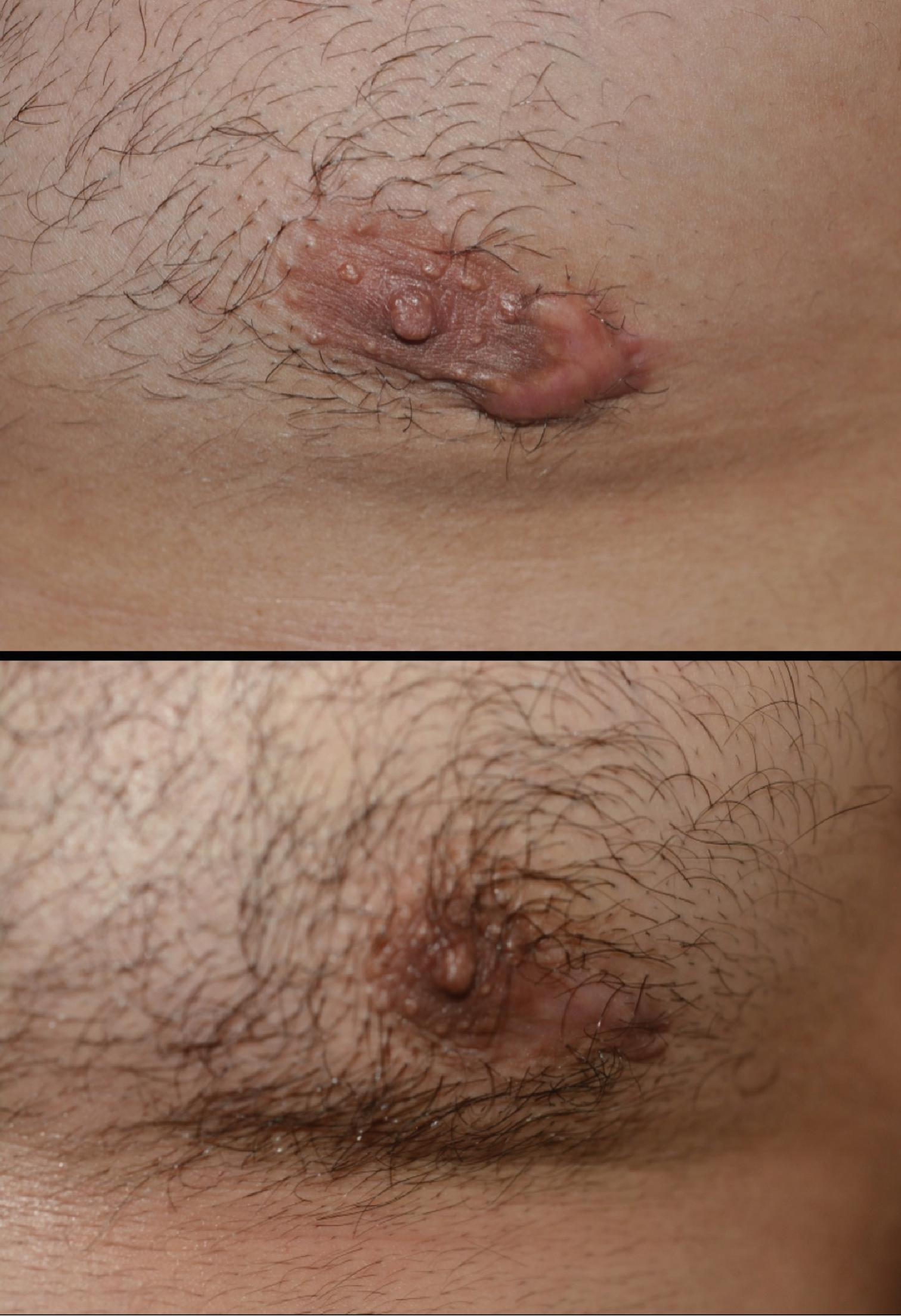
Compression therapy may be utilized to minimize erythema and scar thickness. This is thought to cause local tissue hypoxia resulting in fibroblast degeneration and reduction of collagen fiber cohesion. Furthermore, activation of mechanoreceptors results in apoptosis. Options for compression treatment include pressure earrings, elastic adhesive bandages, ACE bandages, spandex or elastane, and support bandages. A study showed that 60% of patients treated with compressive devices demonstrated 75% to 100% improvement. Button compression dressings used for earlobe keloids prevented recurrence over a period of 8 months to 4 years in one study. One published protocol for earlobe keloids post excision resulted in a recurrence rate of 29.5% after using pressure clips for 12 hours a day between 6 and 8 months. These interventions are frequently limited by patient discomfort due to the need to provide adequate continuous pressure to the wound throughout most of the day for several weeks ( Fig. 8.3 ).
Tacrolimus has potential therapeutic targets for keloid treatment. In vitro studies have shown its ability to block the TGF-β/Smad pathway in keloid fibroblasts by downregulating TGF-β receptors. This results in reduction of keloid fibroblast proliferation, migration, and collagen production. Furthermore, tacrolimus is known to bind the Gli1 oncogene that may be implicated in keloid pathogenesis. One study of 11 subjects treated with tacrolimus 0.1% ointment twice daily for 12 weeks reported patient improvement in tenderness, induration, pruritus, and erythema.
Retinoids are also able to improve hypertrophic scar (HTS) and keloid scars through their inhibition of matrix metalloproteinases. A study of 11 subjects using tretinoin 0.05% nightly for 12 weeks reported a significant decrease in both the weight and size of the scars. In terms of prevention, a study comparing tretinoin cream and silicone gel versus a control group showed successful prevention of HTS and keloids with the former interventions. They also reported improvement in the final scar appearance.
Onion extract is available over the counter and contains the active ingredient allium cepa. Its derivative, quercetin, has antiinflammatory properties via mast cell stabilization and antiproliferative effects. Interestingly, elevated histamine levels result in increased collagen synthesis by fibroblasts. Studies have demonstrated elevated histamine levels in keloid and hypertrophic scars compared to normal skin. Randomized controlled trials have demonstrated that onion extract is most useful when used in combination with other therapies such as with silicone gel sheets or intralesional corticosteroids. Patients experienced improved pain, itching, and reduced elevation when combined with intralesional triamcinolone and improved color and height when combined with silicone gel sheets.
Radiation Therapy
Radiation has a role in the treatment of keloids and involves relatively short durations such as 25 Gy/5 fractions for 5 days. However, it should be noted that there are serious risks to consider including radiation-induced carcinogenesis. Radiation should be avoided in children and infants as well as the thyroid and mammary gland areas. It should also be judiciously used in darker skin types as it may cause permanent hypopigmentation.
SURGICAL INTERVENTION FOR THE MANAGEMENT OF PATHOLOGIC SCARS
About 100 million patients develop scars worldwide, annually. About 55 million of these are from elective procedures and 25 million are from trauma, including burns (∼500,000). Scar revision for cosmetic and/or functional complaints was the fourth most commonly performed reconstructive procedure in plastic surgery in 2017. In the case of scars that are causing functional impairment (e.g., contracture in scars crossing over joints or spanning mobile body sites, such as the neck), surgical intervention is often a key component of multimodal scar revision.
In the case of pathologic scars, particularly keloids, surgical excision monotherapy (almost) always results in recurrence. Many of the scar treatment modalities discussed in this chapter, however, can be used as adjuvant therapy after surgical excision to optimize outcomes and reduce risk of therapy failure. Intralesional corticosteroid injections, for example, are one of the more commonly utilized postoperative adjuvant interventions and have been shown to decrease risk of keloid recurrence.
When HTS are resistant to less invasive treatments, they can be surgically excised, being careful to employ surgical techniques that optimize tension vectors and minimize strain on the new scar. Some patients may also benefit from scar reorientation, lengthening, or tension release, through techniques such as Z-plasty, W-plasty, and V-to-Y advancement flaps ( Fig. 8.4 ). In cases of larger keloids (and full-thickness involvement of sites like the ears), partial excisions may be the only practical strategy. Literature is inconclusive regarding preferred suture type to mitigate risk of recurrence—one randomized controlled trial study suggests there is no difference when absorbable versus nonabsorbable sutures were used. Another study showed that nonabsorbable sutures may reduce risk of HTS recurrence. Regardless, surgical techniques should prioritize minimization of dermal tension and subsequent prolonged inflammation (e.g., flaps, Z-plasty, subcutaneous and deep fascial plication sutures). A proposed excision protocol for earlobe keloids involves dissecting out the keloid, taking care to preserve as much overlying epidermis as possible. This allows for suturing achieving minimal dermal tension and judicious placement of deep dermal sutures. Complete top stitching with either nonabsorbable or absorbable sutures should be performed in a simple interrupted manner followed by closure with adjunct intralesional steroid. Close patient follow-up for repeat intralesional steroid injection and reassessment is recommended between 2 and 4 weeks.