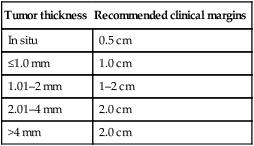
Staging of melanoma is crucial because it not only assigns patients into well-defined risk groups, it also aids in clinical decision-making as reviewed in the latest National Comprehensive Cancer Network (NCCN) clinical practice guidelines on melanoma. The goal of surgical management of primary cutaneous melanoma is to achieve negative histological margins to prevent recurrence and metastases. The current surgical margin guidelines from the NCCN are based on Breslow depth (Table 143.1). The standard treatment for stage IA melanoma is wide local excision. Total excision of primary melanoma with wide margins offers the best chance for cure. However, melanoma cells may extend non-contiguously for several millimeters beyond the visible lesion. Table 143.1 Current melanoma excisional margin guidelines Coit D, et al. Available online: http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf
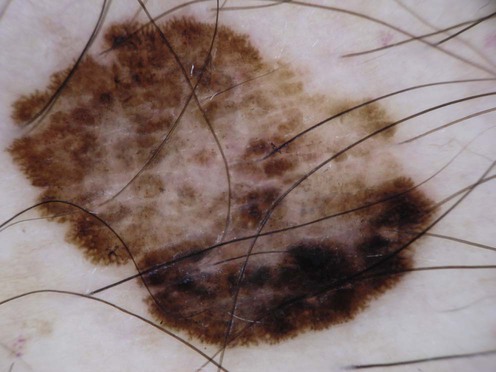
Malignant melanoma
Management strategy
Tumor thickness
Recommended clinical margins
In situ
0.5 cm
≤1.0 mm
1.0 cm
1.01–2 mm
1–2 cm
2.01–4 mm
2.0 cm
>4 mm
2.0 cm
Specific investigations
National Comprehensive Cancer Network. Clinical practice guidelines in oncology. V 1.2013.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








