Fig. 4.1
Sagittal T2-weighted, fast spin echo (TR/TE 342/150 ms) magnetic resonance image of the pelvis showing the rectum (R), the anal canal (A), and their relation to the prostate (P) and bladder (B). S pubic symphysis, S1 first sacral bone
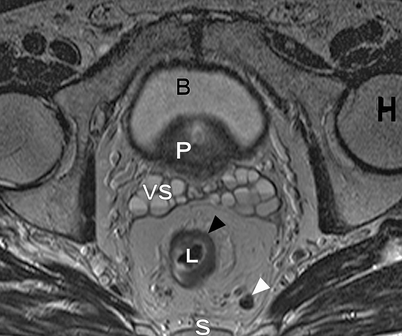
Fig. 4.2
Axial T2-weighted, fast spin echo (TR/TE 342/150 ms) magnetic resonance image of a male pelvis. This image shows the close relation of the organs in the pelvis. On the posterior side, the sacral bone is visible (S); on both lateral sides, the head of the femur (H) is visible. The lumen of the rectum (L) is hypointense. The rectum itself contains an isointense circular rectal tumor (black arrowhead). On the anterior side of the rectum with its surrounding fat, the seminal vesicles (VS), prostate (P) and bladder (B) are located. The mesorectum of this patient with rectal cancer also contains a lymph node (white arrowhead)
Mesorectal Compartment
The mesorectal fascia (Fig. 4.3) envelops the mesorectal compartment containing the rectum and the mesorectal fat comprising blood vessels lymph nodes and lymphatic vessels. This region is referred to as the mesorectum. The normal rectal wall is depicted as two layers on T2-weighted magnetic resonance images. The hypointense layer corresponds to the muscularis propria recti and the inner isointense layer represents the mucosa. When the rectal wall is inflamed (i.e., in inflammatory bowel disease or as a reaction to radiotherapy), it becomes edematous, which is depicted by a third layer with high signal intensity between the inner and outer layer (Fig. 4.4).
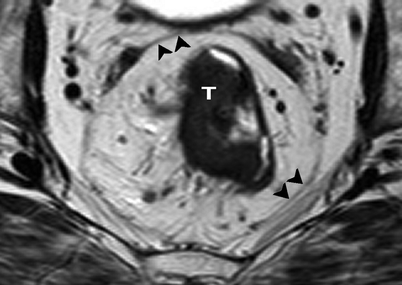
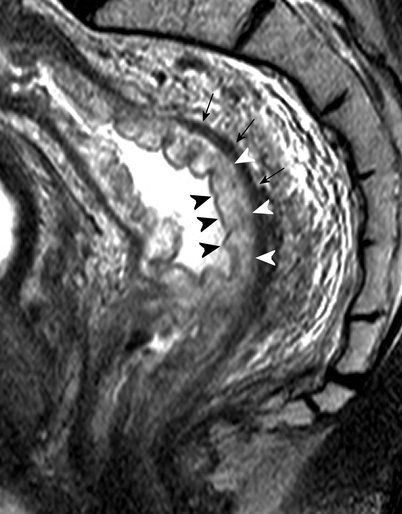

Fig. 4.3
Axial T2-weighted, fast spin echo (TR/TE 342/150 ms) magnetic resonance image of the pelvis. Rectal tumor (T) and surrounding mesorectal fat are enveloped by the hypointense mesorectal fascia (black arrowheads)

Fig. 4.4
Sagittal T2-weighted, fast spin echo (TR/TE 3427/150 ms) magnetic resonance image of a male patient with rectal cancer shows a high signal-intensity layer (white arrowheads), indicating submucosal edema. This layer is situated between the inner mucosal layer (black arrowheads) and the muscularis propria recti (black arrows) (Reprinted with permission from Lahaye et al. [1])
On T2-weighted magnetic resonance images, the mesorectal fat is seen as a high signal intensity structure. The mesorectal fat surrounds the rectal wall but does not have the same thickness throughout its length and circumferential location. On the anterior side, the layer of mesorectal fat is thinner; therefore, the anterior rectal wall is in close proximity to the genital organs, which consist of the prostate and seminal vesicles in men and the vagina and cervix in women. In rectal cancer, this close relationship as well as the distal tapering of the mesorectal fat makes invasion of the anterior organs as well as the pelvic floor more likely (Fig. 4.5).
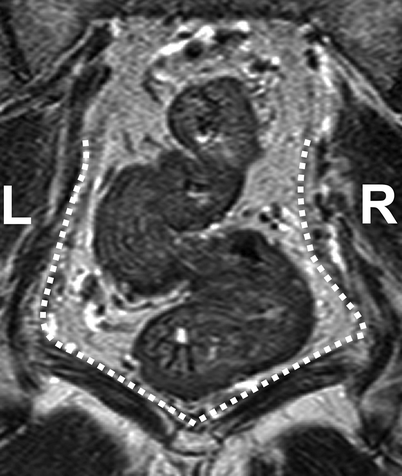

Fig. 4.5
Coronal T2-weighted, fast spin echo (TR/TE 3427/150 ms) magnetic resonance image of a patient with rectal cancer showing the distal tapering of the mesorectum, which entails a close relationship of the distal rectal wall and the muscles of the pelvic floor (white dotted line) (Reprinted with permission from Lahaye et al. [1])
The mesorectal fascia itself is depicted on T2-weighted magnetic resonance images as an hypointense fine line surrounding the mesorectal fat (see Fig. 4.3) [2]. Anteriorly, the fascia becomes thicker at the point where it separates the fat from the seminal vesicles in men and the vagina in women. At this point, the fascia is referred to as Denonvilliers’ fascia. Posteriorly, the presacral fascia of Waldeyer, visible as a hypointense signal, is located between the mesorectal fascia and the sacrum. During surgical resection of the rectum, the plane between these two fasciae is the plane of resection. The upper two thirds of the (meso)rectum are enveloped by the peritoneum on the anterior and lateral sides. Anteriorly, the peritoneum is reflected at the height of the seminal vesicles in men and the cervix/posterior vaginal wall in women to form the rectovesical or rectouterine pouch (of Douglas).
Anal Sphincter
The muscular part of the anal canal is formed by the internal and external anal sphincters, with a longitudinal muscle layer lying in between. More proximally, this sphincter complex is surrounded by the levator ani complex, which forms an important part of the pelvic floor (Fig. 4.6). The main innervation of the levator ani complex and the external anal sphincter is the pudendal nerve. The internal anal sphincter is innervated by the parasympathetic nerve fibers arising from S2 to S4.
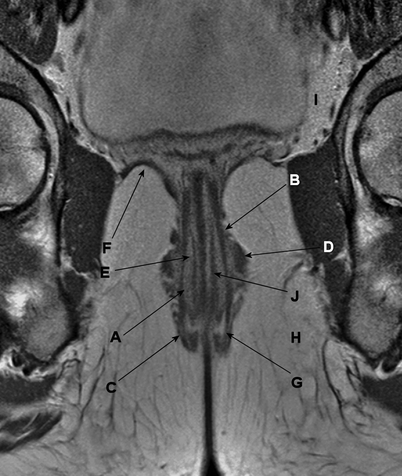

Fig. 4.6
Coronal gadolinium contrast–enhanced, T1-weighted, fast spin echo magnetic resonance image. The muscles of the anal canal are indicated: (a) the internal sphincter, (b) the longitudinal muscle layer, (c) the external sphincter, (d) puborectalis muscle, (e) m. submucosae ani, (f) levator ani muscle, (g) intersphincteric space, (h) ischioanal (or ischiorectal) space, (i) supralevator space, and (j) thin muscle layer located within the submucosa
Vascular Supply
The main blood supply of the rectum arrives via the superior rectal artery, arising from the inferior mesenteric artery. The superior rectal artery divides into a right and left branch, feeding both sides of the rectum. The superior rectal vein runs dorsally parallel to the artery. The superior rectal artery is depicted as a hypointense structure on T2-weighted MRI (Fig. 4.7). The distal part of the rectum also is supplied by the middle rectal artery, arising from the internal iliac artery. The internal iliac artery and vein are situated on the lateral pelvic sidewall. The inferior rectal artery arises from the pudendal artery, whereas the latter is a branch of the internal iliac artery. For surgeons, the presence of the presacral venous plexus, situated just behind Waldeyers’ fascia, is of importance because accidental injury can lead to profuse bleeding with great difficulty in hemostasis.
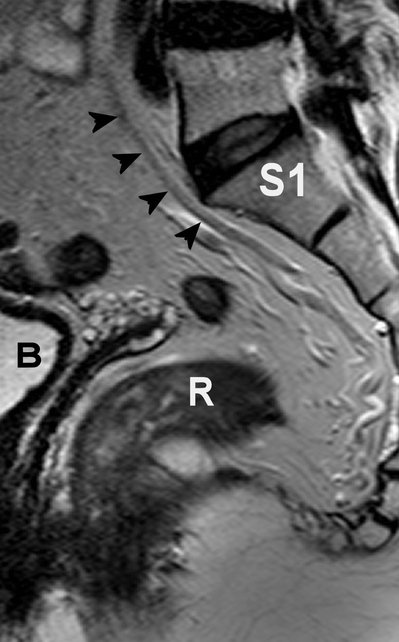

Fig. 4.7
Sagittal T2-weighted, fast spin echo (TR/TE 3427/150 ms) magnetic resonance image of the pelvis. The hypointense superior rectal artery is indicated by black arrow heads. R rectum, B bladder, S1 first sacral bone
Lymphatic Drainage
The main lymphatic drainage of the rectum follows the superior rectal vein and the inferior mesenteric vein to the para-aortic nodes. Mesorectal lymph nodes are mainly situated in the lateral and posterior part of the mesorectum [3]. Further lymphatic drainage of the rectum, especially the low rectum, is through the drainage system along the middle rectal artery and vein, the so-called lateral pelvic nodes. Nodal metastases do occur in these nodes, usually in low rectal tumors, and most often are associated with involved mesorectal nodes (Fig. 4.8) [4, 5].
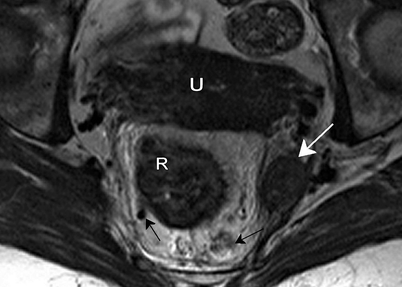

Fig. 4.8
Axial T2-weighted, fast spin echo (TR/TE 3427/150 ms) magnetic resonance image. There is a large lymph node suspected to be malignant outside the mesorectum (white arrow) in the region of the middle rectal artery. There are also nodes located within the mesorectum (black arrows). The left node is not large and has a sharp border and an homogeneous signal, so it could be a benign node; however, the right mesorectal node is large, has an irregular border and a heterogeneous signal, and is therefore a malignant node. R rectal tumor, U uterus
Nerves
Innervation of anorectal, urinary, and sexual function is located in the pelvis. Sympathetic as well as parasympathetic nerves course along the walls of the pelvis in close proximity to the mesorectum, making invasion by tumor in the proximity of the nerves and injury during surgery more likely [6, 7]. The superior hypogastric plexus is located at the level of the sacral promontory, dorsal to the rectum. It divides into left and right hypogastric nerves, which run around the rectum along the pelvic side wall to the bladder and the genital organs. At the level of S2–S5, these nerve fibers are accompanied by parasympathetic fibers, the nervi erigenti, which follow the same route to the bladder and the genital viscera. The sympathetic and parasympathetic nerve fibers together form the right and left inferior hypogastric plexus. This inferior hypogastric plexus cannot be depicted on MRI.
Rectal Cancer
Colorectal cancer was diagnosed in almost 300,000 patients in Europe in 2006. It is the third most common cancer in men and women taken together and the second most common cause of cancer-related death [8, 9]. About 30 % of all colorectal cancers are located in the rectum. Apart from cancer-related death, rectal cancer is especially notorious for local recurrence, which is accompanied by severe morbidity. The treatment of rectal cancer, therefore, focuses on optimal local and distant control. In past decades, this treatment has undergone major developments both in surgery and neoadjuvant therapy. With regard to surgery, the introduction of total mesorectal excision (TME) has significantly reduced the local recurrence rate [10]. In TME, the rectum is removed along with its surrounding fat, including lymph nodes and blood vessels, through which tumor cells can spread, using a sharp surgical technique along the outside of the mesorectal fascia, creating a mesorectal package. This improvement in surgical technique was driven by the notion that the distance of the tumor to the circumferential resection margin (CRM), as judged by the pathologist, was an important factor in determining local recurrence rates [11]. At the same time, Scandinavian trials have shown that preoperative radiotherapy in addition to conventional surgery is more effective than postoperative radiotherapy for reduction of local recurrence. The Dutch TME trial [12] as well as the MRC CR07 trial [13] further showed that, even with good TME surgery, neoadjuvant 5 × 5 Gy short-course radiation therapy has a favorable effect on local recurrence rates for the whole group; hence, in the Netherlands and Scandinavia, 5 × 5 Gy before TME has become the standard treatment for all patients with rectal cancer. A subgroup analysis of the Dutch TME trial also showed that a short course of preoperative radiation (5 × 5 Gy) does not improve outcome in stage I tumors (T1-2N0) because these tumors already have a favorable prognosis without radiotherapy [12]. Because it was a subgroup analysis and because of the difficulties with nodal staging, clinicians were reluctant to withhold radiotherapy from these patients. On the other hand, for more advanced tumors – those with an involved CRM – it has been shown that the recurrence rates were too high, even after a preoperative short course of radiation [14]. To avoid local recurrences in this group, more aggressive neoadjuvant treatment has been advocated. In 1991, Krook et al. [15] showed the advantage of the addition of chemotherapy to adjuvant radiotherapy for reduction of local recurrence and the improvement of overall survival of patients with more advanced tumors. Two other studies proposed chemotherapy plus radiation therapy in a neoadjuvant setting because it not only improved the local control, [16, 17] but also showed a better adherence to treatment when compared with postoperative chemotherapy [16]. In 2004, Sauer et al. [18] confirmed that this combined chemoradiation is more effective in terms of local control and toxicity when given preoperatively compared with postoperatively. Locally advanced rectal cancer is, therefore, preferably treated with long-course neoadjuvant radiation, with chemotherapy as a radiosensitizer.
The selection of these “more advanced tumors” with a high risk for circumferential margin involvement has long been made based on clinical examination (“fixed tumors”) and endosonography, when available. Clinical and endosonographic staging is not very accurate for assessing tumors with involved CRMs. In the Dutch TME trial, where the intent was to exclude clinically fixed (“locally advanced”) tumors, there was a rate of involved margins of 16 %, often due to the inclusion of large “advanced tumors” that were understaged. With the introduction of MRI at the beginning of this millennium to the workup of patients with rectal cancer, the mesorectal fascia – the anticipated CRM – could accurately be visualized. Tumors that were growing into or near the mesorectal fascia could be better identified with this approach [2, 19–24]. This has been a significant development because of the importance of the circumferential resection plane, which had been previously established [11].
Apart from the CRM, the presence of nodal disease is an important risk factor for local recurrence. Until recently, imaging modalities lacked sufficient accuracy for nodal prediction because size was the main predictive criterion, and in rectal cancer, lymph node micrometastases have been known to occur in nodes with a maximal diameter smaller than 5 mm [25]. The introduction of lymph node–specific contrast agents for MRI offered the hope of better lymph node prediction [26]. These developments in MRI related to accurate prediction of the CRM as well as promising results with regard to nodal staging could lead to a more differentiated treatment approach in rectal cancer rather than one uniform treatment for all. Of all available treatment options, one that best fits an individual patient can be chosen on the basis of an assessment of the risk for local recurrence. Theoretically, this differentiated treatment leads to optimal local control with minimal treatment-related morbidity for the whole group of rectal cancer patients.
MRI-based, tailored treatment of rectal cancer and its surgical outcomes have been studied in a prospective multicenter study in the Netherlands; the definitive results of the 3-year local recurrence rate will soon be available. In this study, all rectal cancer patients received preoperative MRI, on which the important risk factors for recurrence (i.e., CRM, nodal status, and tumor height) were identified. Based on the MRI findings, stratification was performed as follows: surgery alone for the low-risk cases, 5 × 5 Gy radiotherapy plus surgery for the intermediate risk cases, and a long course of preoperative chemoradiotherapy (CRT) for the high-risk cases. The purpose of this study was to show that differentiated treatment for patients with rectal cancer provides optimal treatment with minimal treatment-related complications. The preliminary results of this study show an actuarial 2-year local recurrence rate of 2.8 %, which proves that this differential treatment is safe [27].
Today, however, other studies are exploring even more directed treatment, exploiting the sometimes phenomenal responses to neoadjuvant CRT. Complete response rates of up to 25 % have been described, and an even larger proportion of patients have considerable downsizing or downstaging [28]. The current trend is toward minimally invasive, organ-sparing surgery in the good responders after CRT and even a wait-and-see policy in the complete responders [29–31]. The accuracy of using MRI for restaging tumors after CRT is of great importance in this scenario.
Staging of Rectal Cancer with MRI
T Stage
The T stage of rectal cancer is divided into four categories. A T1 tumor is confined to the submucosa; T2 refers to a tumor that invades the muscularis propria, T3 invades the mesorectal fat, and T4 invades surrounding organs. The accuracy of MRI for the prediction of T stage shows a wide variability in different studies, ranging from 67 to 83 % [32]. This wide range is partly explained by the inaccuracy of MRI in reliably distinguishing between T1 and T2 tumors because the submucosal layer is not depicted. For this distinction, endorectal ultrasound is more reliable [32]. The other reason is the difficulty of MRI to distinguishing between T2 and T3 tumors. With an intact hypointense muscular wall, the tumor most often does not invade the mesorectal fat (positive predictive value, 86–91 %) [33]. However, in cases of desmoplastic reaction in borderline T2 or T3 tumors, MRI has difficulty in distinguishing accurately between desmoplastic reaction with (pT3) or without (pT2) viable tumor cells (Fig. 4.9a, b). However, large T3 tumors and T4 tumors can be accurately selected using MRI, with sensitivities of 74 and 82 % and specificities of 76 and 96 %, respectively [34].
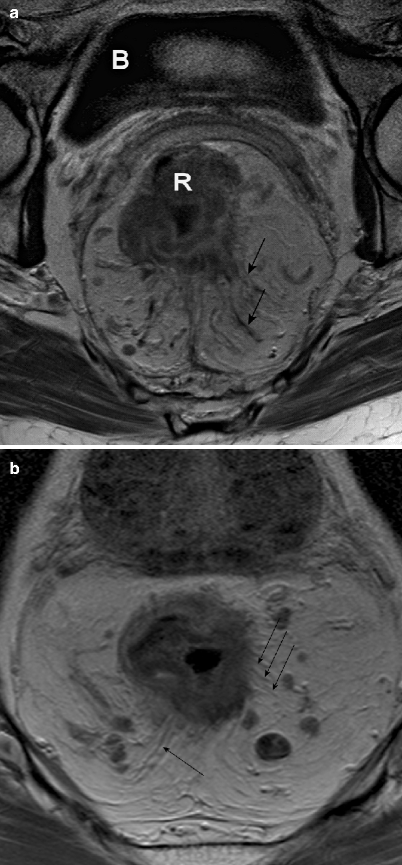

Fig. 4.9
(a and b), Axial contrast-enhanced, T1-weighted, turbo spin echo (TR/TE 612/15 ms) magnetic resonance images. Both show a desmoplastic reaction to a rectal tumor (black arrows), although it is impossible to differentiate between desmoplastic reaction with (b) tumor cells (therefore a T3 tumor) and desmoplastic reaction without (a) tumor cells (therefore, a T2 tumor). Note: Both images also show mesorectal lymph nodes. R rectal tumor, b bladder (Reprinted with permission from Beets-Tan et al. [21])
Circumferential Resection Margin
The mesorectal fascia – the surgeon’s plane of resection in rectal cancer surgery – is clearly depicted as a hypointense line on transverse T2-weighed MRI. Therefore, the CRM can be accurately measured and predicted. This has been shown in a meta-analysis of seven single-center studies, with sensitivities of 60–88 % and specificities between 73 and 100 % [1], and has been confirmed by a large, prospective, multicenter study by the Mercury Study Group, showing an overall accuracy of 88 %, suggesting that the prediction of the CRM is reliable in experienced hands (Fig. 4.10) [35].
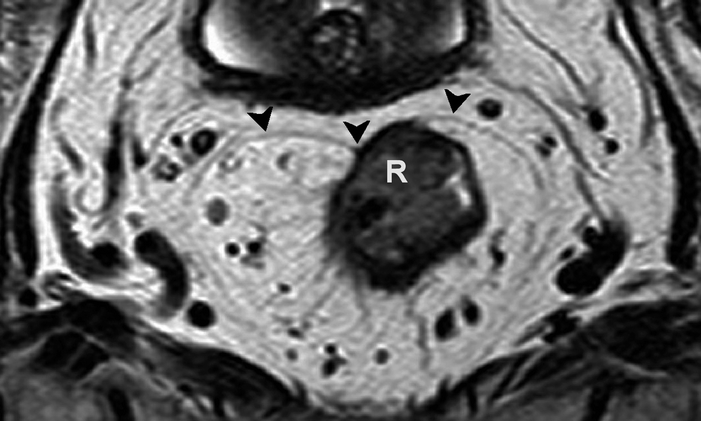

Fig. 4.10
Axial T2-weighted, fast spin echo (TR/TE 3427/150 ms) magnetic resonance image showing a rectal tumor (R) that involves the mesorectal fascia (black arrowheads) on the anterior side









