Fig. 24.1
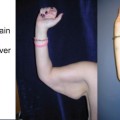
Inguinal lymph node (LN) flap. Superficial lymph nodes are harvested based in the superficial circumflex vessels. Lymph nodes draining lower limb are located medially and centrally to the femoral artery, and should therefore be left intact
Saaristo et al. used similar inguinal lymphatic flap design, with the modification of avoiding harvesting SIEA as a second vascular pedicle. It hypothesized that surgical trauma to this central area might interfere with lymphatic drainage of the lower limbs [13, 23]. In most patients lymph node transfer was combined with the lower abdominal breast reconstruction. They emphasize limiting the lymphatic flap dissection to the lateral border of the femoral artery. Lymph nodes draining lower limb are located medially and centrally to the femoral artery [24], and should therefore be left intact. This is recommended also by Tourani et al. [25].
Dayan et al. describe a slightly more distal flap design. The landmarks for flap are the pubic tubercle, the anterior superior iliac spine, and the line 3 cm below inguinal ligament where the targeted lymph nodes are located based on the magnetic resonance angiography. They also advocate the use of intraoperative indocyanine lymphography to confirm the nodes of interest and reverse lymphatic mapping to avoid harvesting lymph nodes draining the lower extremity [21]. Some other authors report similar below inguinal ligament flap design [15, 20, 22]. Gharb et al. describe a modified technique of harvesting the flap with only the superficial branch of SCIA [26].
Thoracic
The thoracic lymph node flap consists lymph nodes from the lower axilla along the anterior border of latissimus dorsi muscle (Fig. 24.2). According to studies by Suami et al. axillary lymph nodes have separate groups for drainage of the thorax and the arm [27]. Therefore, the lymph nodes of the lower part of axilla could be used for lymph node transfer. The nodes are vascularized either by lateral thoracic vessels or braches of thoracodorsal vessels [16, 28]. The incision is made at the anterior axillary line and dissection is continued through subcutaneous tissue to the lateral border of the pectoralis minor. The lateral thoracic and thoracodorsal vessels are identified and lymph nodes around the appropriate vessels are harvested. According to Dr. Becker, in 60 % of the cases the nodes are supplied by lateral thoracic vessels and these are preferred. In remaining 40 % of the cases the nodes are harvested with the thoracodorsal vessels. It is important to keep the level of harvest inferior to the lateral border of pectoralis minor (level I) to avoid damaging nodes draining upper extremity and breast (level II–III). Reverse sentinel node mapping can be used to identify lymph node draining the arm in order to avoid these during the flap harvest. Prolonged local edema at the donor site has been reported [28] and we have also experienced a postoperative lymphedema of the breast after harvesting the thoracic lymph node flap.
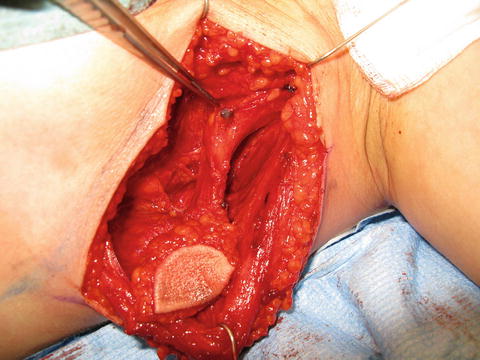

Fig. 24.2
The thoracic lymph node flap containing lymph nodes from the lower axilla along the anterior border of latissimus dorsi muscle
Cervical/Supraclavicular
The cervical lymph node flap [29, 30] is based on the transverse cervical artery and its concomitant transverse cervical vein. External jugular vein is included in the flap to improve venous outflow. It is recommended to be harvested at the right side of the neck to avoid potential damage to the main lymphatic duct. The landmarks of the flap are the clavicle inferiorly, the sternocleidomastoid muscle anteriorly, the trapezius muscle posteriorly, and the external jugular vein. The incision made 1.5 cm above the clavicle and through the plastysma. The omohyoid muscle is retracted and deep to this muscle the TCA and the surrounding lymph nodes are identified and harvested. There are anatomical variations in the origin of TCA. It most often originates from the thyrocervical branch (80 %) or directly from the subclavian artery (20 %), but it may arise as a branch of the internal mammary artery [30]. The main advantage of the cervical lymph node flap is that there is less risk of developing lymphedema at the donor site.
Submental
Submental lymph node flap [31] is based on the submental artery arising from the facial artery. The flap is harvested with an elliptical skin paddle with its upper margin along the lower border of mandible. The facial artery and its junction with the submental artery are identified and the soft tissue around this area containing the lymph nodes is included. The flap is raised along the axis of the submental artery from proximal end to the distal end. The marginal mandibular nerve must be carefully preserved. The anterior belly of the digastricus muscle can be included in the flap for easier harvest.
Lymph Node Transfer to Axilla
Wide scar release is an essential step when preparing the recipient site. All scarred and fibrotic tissue around vessels and nerves is excised and removed if possible. The axillary vein should be completely released to allow free venous outflow (Fig. 24.3). It is recommended to start dissection from the healthy area to identify normal anatomy and avoid injury to the vital structures within the axilla. Dissection should be continued until healthy tissue is again reached. If the patient has chronic pain or symptoms of brachial plexus neuropathy or neuroma is encountered external neurolysis of the brachial plexus should be performed.
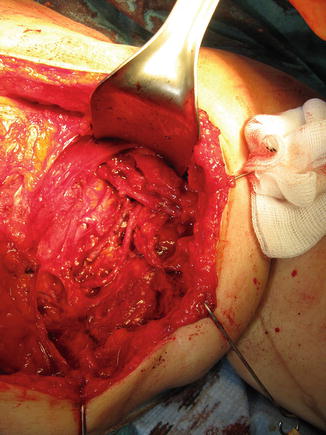

Fig. 24.3
In the upper limb lymphedema patients wide scar release is an essential step when preparing the recipient site for the lymph node flap in the axilla
Recipient vessels, either thoracodorsal or circumflex scapular, are prepared and anastomosed with the flap pedicle using microsurgical technique. Also the lateral thoracic vessels can be used if still available. The lymph node flap is positioned along the axillary vein extending into the proximal brachium.
Lymph Node Transfer to Groin
At the moment there is very few publications about the use lymph node transfer in the inguinal area. Unlike in the breast cancer related lymphedema, the lymphatic vascular problem in the lower extremity is rarely localized only to the proximal area of the limb. According to Becker et al., the principles of lymph node transfer to groin are similar as for that in axilla [16]. Wide scar release is considered important and should be continued until healthy tissue is reached. The superficial circumflex iliac vessels or any local vessels available are used for anastomosis. A pocket just caudal to the inguinal ligament is created to accommodate the lymph node flap.
Some authors favor distal recipient sites (knee or ankle) advocating that “pumping” mechanism of the lymph node flap works better at the distal sites due to gravitational forces [31]. So far, there are no comparative studies of the efficacy of proximal versus distal recipient site. The method is chosen according to the patient’s situation and surgeon’s personal preferences [32].
Combined with Breast Reconstruction
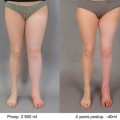
Lymph node transfer can be conveniently combined with microvascular abdominal wall breast reconstruction (Fig. 24.4). For postmastectomy patients suffering from upper extremity lymphedema, breast reconstruction with lymph node transfer is an optimal choice. Any soft tissue flap from the lower abdominal wall, either based on the deep inferior epigastric vessels (traditional TRAM-flap, muscle spearing TRAM-flap or DIEP flap) or superficial ineferior epigastric vessels (SIEA flap) can be used as a carrier of lymph node flap. The superficial lateral lymph nodes are located just caudal to this flap, and therefore, the skin incision in the combined flap is placed slightly lower (in a W-fashion) than when performing only abdominal flap (Fig. 24.4). The dissection starts with identification of SCIA and SIEA/V. The lymph node flap is dissected from lateral to medial following SCIA or its perforator and the pedicle vessel are ligated at their origin. The lymph node flap stays in connection to the abdominal flap at this level. Dissection should be limited to the lateral border of the femoral artery not to damage lymphatic tissue draining the lower limb, and SIEA and SIEV should be dissected with care. Abdominal flap is then elevated as TRAM flap, ms-DIEP, DIEP or SIEA flap depending on the patient’s anatomy and surgeon’s preference. Before ligating the main pedicle vessels, blood flow to the tip of the lymph node flap is assessed. Perioperative ICG angiography can be useful when evaluating the blood perfusion to the lymph node flap. Axillary recipient site is prepared as described earlier. The main pedicle of the abdominal flap in anastomosed to the thoracodorsal vessels [13] or the internal mammary vessels [16]. If blood perfusion to the lymph node flap is not adequate, the SCIA/V are anastomosed to the retrograde thoracodorsal vessels or to circumflex scapulae vessels [12, 33] or to intact thoracodorsal vessels in case of internal mammary vessels were used for the main anastomosis [16]. The lymph node flap is the placed into the pocket along the axillary vein extending to the proximal brachium and the abdominal flap is shaped to reconstruct the missing breast.
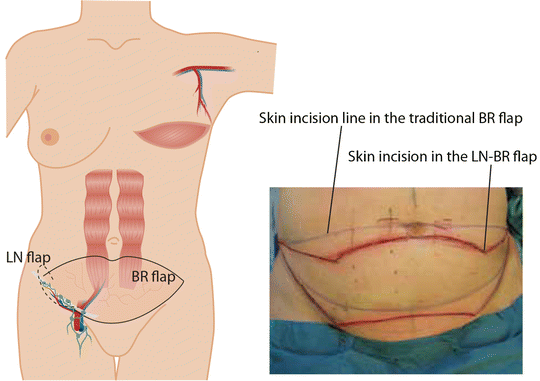

Fig. 24.4
Lymph node transfer can be conveniently combined with microvascular abdominal wall breast reconstruction (a TRAM, msTRAM, DIEP, or SIEA flap reconstruction). The superficial lateral lymph nodes are located just caudal to the lower abdominal wall breast reconstruction flap, and therefore, the skin incision in the combined flap is placed slightly lower (in a W-fashion) than when performing only abdominal flap
Postoperative Care
There is no clear consensus of the optimal postoperative treatment.
Becker et al. start physiotherapy on the first postoperative day and continue it daily up to 3 months. After that physiotherapy is done two times a week for the next 3 months and then discontinued. Compression therapy is not used postoperatively [10].
It is our practice to start manual lymphatic drainage as soon as possible postoperatively to theoretically support the spontaneous regrowth of the lymphatic vasculature in the axilla. From the experimental studies we know that lymphatic vascular growth and maturation process may take 2 to 6 months after surgical operation [34]. However, there are no clinical studies to support this practice. The compression therapy is always continued minimum 6 months after the surgery. After 6 months we encourage patients to stop using the compression garments. However, most patients still need to use compression also after that at least in physically strenuous situations. Depending on the extent and duration of preoperative lymphedema, compression may be needed up to 2 to 3 years, or permanently.
Complications
When using the groin area as a donor site, the risk of developing postoperative iatrogenic lower limb lymphedema is a major concern. Iatrogenic upper limb lymphedema is a potential risk also when using thoracic lymph nodes.
Postoperative seroma seems to be a common problem in the inguinal area after the lymphatic flap harvesting. However, that problem can usually be managed by local compression and repeated needle aspirations. Vignes et al. report a series of 26 patients who underwent VLNT for treatment of upper or lower limb lymphedema. Thirty-eight percent of them developed complications including chronic lymphedema at the donor site, lymphocele, testicular hydrocele, and persistent donor-site pain [35]. In a study by Viitanen et al. 4 of 13 patients complained postoperative numbness or pain in the superficial femoral cutaneous nerve area [23]. Pons et al. report a case of donor-site thigh lymphedema after lymph node–superficial circumflex iliac artery perforator flap transfer among 42 patients of VLNT operation [36].
Viitanen et al. evaluated the donor-site lymphatic function in the lower limb after microvascular lymph node transfer by postoperative lymphoscintigraphy. In six of ten patients, the lymphatic flow was slightly slower in a donor-site limb compared with non-operated limb, although none of these patients had clinical symptoms of lymphedema [23]. This emphasizes the importance of postoperative follow-up to detect possible developing postoperative lymphedema. Azuma et al. recommend ICG lymphography for following donor-site lymphatic function [37, 38].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree