Autoimmune connective tissue disorders
Cutaneous lupus erythematosus
Dermatomyositis
Mixed connective tissue disease
Nephrogenic fibrosing dermopathy
Scleroderma (Systemic sclerosis)
Autoimmune mucocutaneous blistering diseases
Bullous pemphigoid
Epidermolysis bullosa acquisita
Lichen planus pemphigoides
Linear IgA bullous disease
Mucous membrane (Cicatricial) pemphigoid
Paraneoplastic autoimmune multiorgan syndrome (a.k.a. Paraneoplastic pemphigus)
Pemphigoid (Herpes) gestationis
Pemphigus foliaceus
Pemphigus vulgaris
Vascular disorders
Anti-neutrophil cytoplasmic autoantibody (ANCA) positive vasculitides:
Microscopic polyangiitis
Wegener’s granulomatosis
Behçet’s disease
Churg–Strauss syndrome
Cutaneous polyarteritis nodosa
Degos’ disease
Leukocytoclastic vasculitis
Livedoid vasculopathy
Drug-induced skin disorders
Drug reaction eruption with eosinophila syndrome/Anticonvulsant hypersensitivity syndrome
Erythema multiforme
Kaposi sarcoma due to immunosuppression
Methotrexate-induced acral erythema
Stevens–Johnson syndrome
Toxic epidermal necrolysis (Lyell’s syndrome)
Miscellaneous dermatoses
Alopecia universalis
Atopic dermatitis
Calcinosis cutis
Chronic urticaria:
Angioedema with hypereosinophilia
Autoimmune urticaria
Chronic idiopathic urticaria
Delayed pressure urticaria
Solar urticaria
Graft-versus-host disease
Hyper-IgE syndrome
Kawasaki’s syndrome
Necrobiosis lipoidica diabeticorum
POEMS syndrome: polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes
Polymorphous light eruption
Pretibial myxedema
Psoriasis
Pyoderma gangrenosum
Scleromyxedema
Wiskott–Aldrich syndrome
Skin infectious and infection-related diseases
Lyme disease
Measles
Necrotizing fasciitis
Rubella
Staphylococcal scalded skin syndrome (SSSS; Ritter’s disease)
Streptococcal toxic shock syndrome (STSS)
Varicella
In contrast to standard immunotherapy for autoimmune and inflammatory conditions, IVIg is not immunosuppressive. It is also safe to use in pregnancy and is not associated with reproductive organ suppression, nor is it carcinogenic. It is generally accepted that the use of IVIg should be reserved for those cases that: (1) fail conventional therapy; (2) have severe side effects or contraindications to conventional therapy; and/or (3) have rapidly progressive disease.
IVIg exhibits several effects with the most well described being: (1) complement blockade and degradation; (2) neonatal Fcγ receptor saturation; (3) induction of immunomodulatory Fc receptors; (4) inhibition of B cells; and (5) altering T cell function, cytokine production and migration.
IVIg is given intravenously over several hours, gradually increasing the rate of infusion up to 200 ml/h. It is given daily for 2–5 days usually at 400 mg/kg/day up to 2 g/kg per month. The dose can be repeated in 2–4 weeks, depending on the disease and patient. Its half-life is 3–4 weeks. Multiple cycles are usually required with up to 50 or more sometimes needed. Table 50.2 outlines the standard infusion protocol for the administration of IVIg. IVIg can be administered in a hospital if there is extensive disease, concomitant very high steroid doses, or other serious medical problems. More commonly, it is administrated at an infusion center or at a patient’s home under medical supervision.
Table 50.2
Standard IVIg infusion protocol
Administer IVIg product at 400–500 mg/kg/day on 4–5 consecutive days up to the total dose of 2 g/kg/month times 6–18 months |
Premedicate patient with 25 mg Benadryl (diphenhydramine) and 500 mg Tylenol (acetaminophen) 15–30 min prior to starting the infusion |
Place peripheral i.v. and maintain with 0.9 % sodium chloride |
Infusion Rate: start at 0.5 ml/kg/h, then increase by 15 ml/h every 15 min until target rate of 150–200 ml/h, as tolerated. Maximum rate is 200 ml/h |
Observe vital signs prior to infusion. Blood pressure and pulse every 30 min until stable infusion rate, then every hour |
Watch for signs of fluid overload, cardiovascular symptoms, allergic reactions, skin rash, fever, and moderate to severe headache |
For adverse events, stop the infusion. Can restart the infusion at the same or lower rate if the symptoms subside |
The annual cost of multiple infusions of IVIg in patients with autoimmune bullous diseases is estimated to be between $33,000–$85,000. This may seem high initially however; the cost is significantly less than the cost of conventional therapy when factoring in management complications and adverse events associated with conventional immunosuppressive therapy [1]. The cost nevertheless results in extensive scrutiny by health insurance companies, requiring significant evidence that several other therapies have failed. Even when approved, the length of treatment may still be restricted.
IVIg is a relatively safe and well-tolerated therapy. Severe adverse events associated with the use of IVIg are rare. Most side effects can be minimized by decreasing the rate of infusion and by giving it over 4–5 days. Additional measures to decrease adverse events include modifications in dilution and using preservative free preparations. Not all IVIg preparations are equal with formulations available in the United States differing in immunoglobulin A (IgA) content, need for reconstitution, method of viral inactivation, osmolarity and sugar content.
In a study of 9892 infusions given to 174 patients, the most commonly reported adverse event was headache, which occurred in 8.9 % of infusions and was reported more often in patients with a history of migraines [2]. Acute self-limited cutaneous reactions such as urticaria also occurred in a small subset of patients.
Other immediate and delayed side effects can be seen with IVIg. Immediate effects include flushing, chills, fever, nausea, vomiting, dizziness, sweating, hypertension, chest pain, back pain, and muscle aches. These are related to the infusion rate and can be minimized by decreasing the rate. Headache and acute cutaneous reactions, such as urticaria, are generally best managed with prophylactic pre-medication with acetaminophen and diphenhydramine.
More serious immediate adverse effects include aseptic meningitis, thrombosis and stroke, anaphylaxis and acute renal failure. These events are rarely reported in association with IVIg, however do warrant consideration and close monitoring in high-risk patients. Thromboembolic events have been reported with the use of IVIg. This rare complication is reported to occur when a large dose is administered at a rapid rate, suggesting it might be related to high plasma viscosity [3]. To avoid this complication, high-risk patients should be treated only if necessary and monitored closely. In addition, low dose, low viscosity reconstituted preparations infused at a slow rate can decrease the risk of this serious adverse event. The risk for thromboembolic events is more common in those with a history of cardiac disease, stroke, thrombosis, and advanced age.
Anaphylactic reactions have been reported when IVIg was administered to patients with an IgA deficiency. Current preparations contain less than 2.5 % of IgA. Anti-IgA antibodies produced in patients with IgA deficiency subsequently react with the infused IgA. Evaluation of IgA deficiency prior to administration of IVIg will eliminate this potential complication.
Acute renal failure results from osmotic injury secondary to sucrose. Sucrose accumulates and causes osmotic nephrosis in the renal tubules, leading to reversible renal failure, usually within a week of administration of IVIg. Patients with pre-existing renal insufficiency, diabetes mellitus, older age or those concomitantly taking nephrotoxic agents should have their renal function closely monitored. These patients should receive a sucrose-free preparation administered slowly and at the lowest effective dose.
Delayed side effects include anemia, cardiac insufficiency due to fluid overload, renal insufficiency from immune complex deposition, and viral infections. Anemia is due to anti-ABO antibodies. The risk for infection is decreased with the recent use of detergent treatments and ultrafiltration.
Autoimmune Mucocutaneous Blistering Diseases
Autoimmune mucocutaneous blistering diseases represent a diverse group of rare diseases that involve the mucous membranes and skin. The immunologic basis is well established with each having a unique group of targeted antigens within the epidermis and dermis. The clinical course, presentation, morbidity, and mortality varies greatly within each disease. Conventional treatment for autoimmune blistering diseases is centered around the use of systemic steroids and immunosuppressive agents. These agents can cause serious side effects, some of which may be fatal.
More recently, IVIg has emerged as an important agent in the treamtent of blistering diseases. An expanding number of reports on the efficacy of IVIg in autoimmune cutaneous blistering disorders (pemphigus vulgaris, pemphigus foliaceus, bullous pemphigoid, mucous membrane pemphigoid, linear IgA dermatosis, epidermolysis bullosa acquista; paraneoplstic autoimmune multiorgan syndrome) parallel its increased utilization. More is known about the use of IVIg in pemphigus and pemphigoid than in any other autoimmune cutaneous blistering disease.
Pemphigus antibodies target several antigens on the cell surface of keratinocytes [4, 5]. These antibodies can also penetrate the cell membrane and bind to antigens on the mitochondrial outer membrane, thus triggering the intrinsic apoptosis pathway [6, 7]. Both anti-desmoglein and anti-mitochondrial antibodies are pathogenic because their adsorption abolishes the ability of pemphigus IgG to cause acantholysis in in vitro and in vivo models of pemphigus. Keratinocytes with damaged mitochondria shrink from the lack of energy and because activation of apoptotic cascades leads to collapse of the cytoskeleton. In turn, antibodies to adhesion molecules such as desmogleins prevent cell re-attachment by steric hindrance at the attachment points on nascent desmosomes. The neonatal Fc receptor (FcRn) expressed by keratinocytes is essential for both the disease-causing activities of pemphigus and pemphigoid autoantibodies and the therapeutic action of IVIg [8]. Most recently, we have demonstrated that these receptors can assist pemphigus IgG in reaching mitochondrial targets. Therefore, saturation of these receptors by IVIg may prevent pathogenic antibodies against intracellular antigens to reach their targets.
IVIg is a safe and effective treatment for autoimmune blistering diseases resistant to systemic immunosuppresion [9–11]. Failure of conventional therapy is defined as continued new blister formation, extension of existing lesions or lack of healing while on a moderate dose of prednisone, specifically, up to 1.5 mg/kg/day for at least 3 weeks. However, due to the rarity and severity of these diseases, well designed prospective trails evaluating its use are generally lacking. As a monotherapy, IVIg rapidly controls disease activity in those with pemphigus vulgaris, pemphigus foliaceus, and bullous pemphigoid within a few weeks [12–14]. Reported benefits of IVIg include a improved clinical outcome, decrease in pathogenic autoantibodies, and a steroid sparing effect [9, 12–15, 21]. In cases of pediatric pemphigus, IVIg delays the need for immunosuppression [16].
A recent review indicates treatment for pemphigus ranges from 1 to 2 g/kg/cycle over 2–5 days, at 3–4 week intervals [1]. However, the dose can be lower, and cycles can be extended by several weeks while maintaining effectiveness [17]. The most effective way to use IVIg is unknown though most favor the use of IVIg at a dose of 2 g/kg/cycle. Ahmed and Dahl [17] proposed the use of IVIg at a dose of 2 g/kg divided over 3–5 days every 4 weeks until disease control is obtained. The interval between infusions is then slowly increased to 6, 8, 10, 12, 14 and 16 weeks, and stopped afterwards. If the disease flares, the frequency of infusions is increased until control is obtained and then the tapering regimen is again resumed. A randomized, double-blind, placebo-controlled trial found that patients treated with one cycle of IVIg at 400 mg/kg/day for 5 days stayed on protocol longer without any additional treatment than the placebo group and also had suppression of autoantibodies levels [10]. These findings suggest a single cycle of IVIg maybe effective in the treatment of pemphigus.
Pemphigus and other autoimmune skin blistering diseases appear more likely to respond when IVIg is used concomitantly with additional treatments. When IVIg is used in combination with systemic steroids and/or immunosuppressive agents, a response rate of 91 % was reported, compared to a response of 56 % when it was used as monotherapy [18]. It has been well documented that IVIg causes a rapid decline in pathogenic autoantibody levels. Following this decline there is often a rebound increase. Therefore, the use of an agent that suppresses the rebound increase in pathogenic antibodies should lead to a sustained response to therapy. Indeed, co-administration of a cytotoxic agent improves the ability of IVIg to lower serum levels of pathogenic autoantibodies in pemphigus [19]. The combination of IVIg with rituximab has been shown to induce a sustained remission in the treatment of pemphigus vulgaris [20]. In that study, 11 patients were treated with two cycles of rituximab 375 mg per square meter of body-surface area every 3 weeks followed by once monthly infusions of IVIg at 2 g/kg every fourth week. All had rapid resolution of skin lesions and long-lasting disease remission off all therapy (mean 31 months). This regimen allowed rapid tapering of systemic steroids and immunosuppressive agents within 2 months.
IVIg has also been shown to effectively treat mucous membrane pemphigoid, bullous pemphigoid and epidermolysis bullosa aquisita. Several studies have demonstrated that in severe mucous membrane pemphigoid, IVIg is more effective at resolving lesions, preventing progression, and producing longer remissions than conventional immunosuppressive therapy [12]. A review of 5 cases of patients with bullous pemphigoid, at one institution, found 4 out of the 5 had either partial or complete response to treatment with IVIg in conjunction with an immunosuppressant [21]. In a single prospective study, the use of IVIg at 2 g/kg/cycle every 4 weeks in 10 patients with bullous pemphigoid led to a significant decline in pemphigoid antibodies at 3 months and complete sustained serologic and clinical remission at 11 months [22]. An additional observation showed that these autoantibodies declined at a greater rate when IVIg was combined with immunosuppressive agents compared to its use as monotherapy [23]. Similar results have been observed in the treatment of recalcitrant epidermolysis bullosa aquisita either alone or in combination with oral corticosteroids at doses of 1–2 g/kg [12, 24].
Drug Hypersensitivity Reactions
IVIg is being increasingly used to treat several drug hypersensitivity reactions such as toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS) and drug reaction with eosinophilia and systemic symptoms (DRESS). TEN and SJS are acute and life-threatening mucocutaneous reactions, most commonly due to drugs. These conditions are characterized by fever and full thickness necrosis and detachment of the epidermis. The two conditions differ by the extent of skin involvement, management, etiology and prognosis. TEN is more severe with skin involvement being greater than 30 % and has a mortality rate as high as 50 % [25, 26]. Transcutaneous fluid loss can be large causing significant electrolyte abnormalities, renal insufficiency and hypovolemia. The large amount of cutaneous involvement also puts patients at risk for infection, which can ultimately lead to sepsis.
The exact pathogenesis of TEN and SJS is only partially understood with evidence pointing to a T cell mediated process that leads to keratinocyte apoptosis. TEN is initiated either by non-covalent, direct interaction of a drug with a specific MHC I allotype or by covalent binding of a drug metabolite to a cellular peptide, forming an immunogenic molecule [26]. Treatment of TEN/SJS includes discontinuation of the suspected medication, supportive therapy and transfer to a hospital with intensive care or burn units.
Several studies report successful treatment with IVIg, with mortality rates ranging from 0 to 12 % [27–30]. A randomized trial and two retrospective studies, found that patients treated with IVIg in addition to standard therapy had more rapid disease resolution compared to patients not given IVIg [31–33]. A recent meta-analysis with meta-regression of 13 studies found that IVIg at dosages of greater than 2 g/kg significantly decreases mortality in patients with SJS or TEN [34]. The most accepted total dose for the treatment of TEN and SJS is 3–4 g/kg per day, given early in the disease. In children, somewhat lower doses ranging from 0.5 to 2.2 g/ kg per cycle have been shown to be safe and effective [35]. In TEN and SJS, IVIg is thought to reduce Fas-mediated keratinocyte apoptosis by blocking the binding of FasL to Fas.
The reports on IVIg use in patients with SJS or TEN are inconsistent. A large retrospective analysis of patients in the European Study of Severe Cutaneous Adverse Reactions (EuroSCAR) evaluated the use of corticosteroids, IVIg, and supportive therapy alone, and found that the group treated with IVIg did not have improved mortality when compared to the supportive therapy group [30]. A retrospective analysis of 64 patients at one center found no benefit from IVIg at any dose in the treatment of TEN or SJS/TEN overlap [36]. There were also no differences with regard to delays in treatment or the duration of IVIg therapy.
DRESS is characterized by a widespread cutaneous eruption, eosinophilia and systemic involvement. Its etiology also is not clear but hypothesized to involve drug detoxification enzyme abnormalities with accumulation of reactive drug metabolites, sequential reactivation of herpes viruses, and genetic predisposition [37]. DRESS has a 10 % mortality rate, most commonly from fulminant hepatitis with hepatic necrosis [37]. Initiation of systemic corticosteriods is the mainstay of treatment. Patients have been effectively treated with adjunctive high dose IVIg [38–42]. IVIg can be used as an alternative steroid-sparing agent or in cases unresponsive to systemic steroids. IVIg provides immune protection, has anti-inflammatory properties and increases immunoglobulin levels in patients with this condition [38]. A case report found monotherapy with IVIg successful [38], but a multicenter prospective open study of 6 patients found no benefit [43]. In DRESS, IVIg may provide benefit via anti-viral antibodies in addition to its more general anti-inflammatory and immunomodulatory effects [38, 44].
Cutaneous Lupus Erythematosus
Lupus erythematosus (LE) is a chronic inflammatory autoimmune disorder that can manifest as a systemic disease or be limited to the skin. Several cutaneous subtypes of LE exist, each of which is partly defined by depth of cutaneous involvement. The three most well described cutaneous variants include acute, subacute, and discoid LE. Its etiology is multifactorial with genetic and environmental factors playing a role. Polymorphisms of the major histocompatibility complex leading to increased immune response to self-antigens, deficiencies of complement components, gender, and autoantibodies are all thought to play a role [45, 46]. In regards to autoantibodies, anti-Ro, anti-La, anti-dsDNA and anti-nucleosome antibodies are thought to play a role in skin disease [45]. Autoantibodies lead to increased cell apoptosis and reduced immune tolerence [45]. Dysregulation of T cells may also play a role in the pathogenesis of LE [46, 47].
The initial treatment approach in limited cutaneous LE is photoprotection and the use of topical corticosteroids and calcineurin inhibitors. In widespread, scaring or refractory cases systemic therapy is necessary. For years antimalarials, primarily hydroxychloroquine, have been the gold standard. In patients who fail antimalarials, therapeutic options include oral retinoids, thalidomide, immunosuppressive agents such as mycophenolate mofetil or methotrexate, dapsone, rituximab, clofazimine, sulfasalazine and systemic corticosteroids [48]. IVIg is a consideration in these difficult to control cases as well.
IVIg has been used to successfully treat cutaneous lupus, either as monotherapy or in conjunction with an immunosuppressant, however controlled studies are necessary to fully evaluate its efficacy [49–54]. Three cases described by Lampropoulos et al. [53] highlighted the use of IVIg in the treatment of refractory subacute cutaneous LE. In two of the cases, IVIg at 0.4 g/kg/day for 5 days resulted in rapid improvement of the disease. However in the third case, initial treatment with 2 g/kg IVIg repeated every 4 weeks was not effective and so a single high dose was given, which resulted in greater than 80 % improvement [53]. An open prospective study of 12 patients receiving an initial dose of 1 g/kg body weight followed by 400 mg/kg monthly, for at least 6 months, found that at least five patients experienced complete clearing. Two had a partial response and three had a limited response. The benefit however was temporary in these cases with relapse occurring shortly after stopping [49]. IVIg has also been used to successfully manage lupus profundus, a type of cutaneous LE in which there is deep involvement of the dermis and subcutaneous fat. A case report describes clinical improvement with IVIg given monthly for 6 months followed by 3 month pulses [52].
Several reports describe positive results when using IVIg for the treatment of systemic LE [55, 56]. An observational, retrospective, clinical study of 52 patients treated with at least one cycle of 2 g/kg found complete remission in 28 % of patients, and partial remission in an additional 38 % [57]. IVIg is also helpful in ameliorating myocarditis, lupus nephritis, and lupus-induced bone marrow suppression [58–64]. IVIg treatment is also reported to improve cutaneous findings in systemic LE such as ulcers, urticaria, and malar rash [65, 66]. The optimal dose for treatment of cutaneous LE is yet to be determined. Typical IVIg dosages are 0.4–2 g/kg given monthly for several months. Positive effects are usually observed within a few days, but these often do not persist after IVIg therapy is discontinued. One study of patients with systemic LE achieved remission for over 2 years in 11 out of 18 patients that had an initial response to therapy [57]. Overall, IVIg appears to be a useful adjunctive or alternative therapy in LE cases that do not respond to traditional treatments. Systemic LE patients demonstrate a significant decline of anti−double stranded DNA autoantibodies after treatment with IVIg in the majority of case reports and mouse models [67]. The mechanism is thought to be due to inhibition of B cell receptors, the cells producing the pathogenic autoantibodies, or cytokine signaling [68, 69].
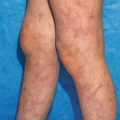
Dermatomyositis
Dermatomyositis (DM) is a multisystem autoimmune disease affecting skeletal muscle, skin and other organs. It most commonly presents as a symmetric proximal extensor inflammatory myopathy and a characteristic photo distributed violaceous cutaneous eruption favoring the face, scalp, and extensor surfaces. There is an amyopathic form in which only cutaneous findings are present, with the muscle disease being absent or subclinical. It has a bimodal distribution with both childhood and adult-onset variants. DM is a complement-dependent microangiopathy mediated by early activation of the complement system with deposition of the C5b-9 membrane attack complex on the endomysial capillaries, which ultimately leads to muscle ischemia and inflammation [70].
First-line therapy for DM is high dose corticosteroids. Although the majority of DM patients respond to corticosteroids, up to 20–30 % will not [71]. In cases that are unresponsive to corticosteroids or a serious steroid related side effect occurs, an immunosuppressant, usually methotrexate or azathioprine, should be started. Both corticosteroids and immunosuppressants are effective in controlling the myopathy, however cutaneous involvement can be particularly refractory to these therapies [72].
IVIg is used as a second line therapy in patients with refractory DM despite treatment with corticosteroids, methotrexate and/or azathioprine, or in those with contraindications to cytotoxic drugs [73]. Several reports justify the use of IVIg early in the course of the disease. IVIg can be considered as a first-line treatment, although IVIg monotherapy has not been shown to be effective [74]. IVIg exerts its action by inactivating immune complexes and decreasing complement amplification. This effect of IVIg was highlighted in vivo in repeated muscle biopsy specimens from DM patients. Membrane attack complex deposits decreased and neovascularization and restoration of muscle cytoarchitecture was observed [75]. In DM, IVIg is usually administered at 2 g/kg over 2–5 days monthly, typically for 3–6 months. If no benefit is noted within 6 months discontinuation of treatment should be considered. The positive outcomes seen with IVIg therapy can be realized for several months after discontinuing therapy. Relapses can occur, however the disease is usually less severe and the need for steroids or immunosuppressive agents is usually decreased.
A double-blind placebo-controlled study by Dalakas et. al assessed the efficacy of 2 g/kg IVIg infusions monthly for 3 months in 15 patients with steroid resistant DM. In the IVIg treatment group, there was significant improvement in muscle strength and active skin lesions [76]. A retrospective review evaluating the effect of IVIg on refractory cutaneous DM in patients with classic and amyopathic DM was performed. All 13 patients treated with 2 g/kg IVIg every 4 weeks (given as 1 g/kg/day for 2 consecutive days) demonstrated improvement, with complete clinical response achieved in 8 [77]. The response to IVIg was very rapid, with all patients improving after the first cycle [77]. IVIg has also been shown to be effective when combined with high dose steroids in treating life-threatening DM associated esophageal dysfunction [78]. More recently, subcutaneous administration of IVIg via a pump, at the usual monthly dose fractionated into equal weekly intervals, was shown to be effective in treating DM and polymyositis [79].
Cutaneous Vasculitis
Cutaneous vasculitis represents a group of disorders characterized by inflammation of small, medium, or large vessels. This condition can be a systemic disease with secondary cutaneous manifestations, restricted only to the skin, or be a primary cutaneous disease with secondary systemic involvement. Systemic findings are broad and nonspecific but commonly include arthralgias, fatigue, hypertension, abdominal pain, renal insufficiency, and neurologic dysfunction. Cutaneous findings are dependent upon the size of the affected vessels. The most common cutaneous manifestations include erythema, purpura, ulcerations, and necrosis. Patients with cutaneous vasculitis often show increased sedimentation rate, anemia, and decreased albumin. The pathophysiology is thought to be due to immune complex deposition in the vessels or anti-neutrophil cytoplasmic antibodies (ANCAs). Immune complex deposition activates the complement system, which in turn leads to mast cell degranulation and neutrophil activation. The end result is vessel wall necrosis. In ANCA mediated vasculitis, cytoplasmic proteins from neutrophils become expressed on the cell surface, leading to antibody formation and then neutrophil mediated vessel damage via release of inflammatory cytokines including IL-1, TNF-α, and IFN-γ, reactive oxygen species, and up regulation of adhesion molecules ELAM-1 and ICAM-1 [80, 81].
Treatment of vasculitis depends on the severity of the disease. In more severe and chronic cases, medium to high-dose glucocorticoids and adjuvant immunosuppressants such as cyclophosphamide or azathioprine are the standard therapy. IVIg is shown to be beneficial in the treatment of organ-specific and systemic vasculitis [82–85]. IVIg has been used to treat ANCA-positive Wegener’s granulomatosis and microscopic polyangiitis [63, 82, 84, 86–92], Churg–Strauss Syndrome [93–98], polyarteritis nodosa [99–106], antineutrophil cytoplasmic antibody-negative nonleukocytoclastic vasculitis [107], Henoch–Schönlein purpura [108], leukocytoclastic vasculitis [109, 110], urticarial vasculitis [111, 112], Behçet’s disease [113], and Kawasaki disease [114, 115].
There is clear evidence that IVIg is beneficial in the treatment of ANCA-positive vasculitides and Kawasaki disease. For the other vasculitidies, there are few studies looking at the efficacy of this treatment. Case reports suggest it may inhibit disease progression [108]. IVIg inhibits TNFα and IL-1 induced proliferation of endothelial cells and reduces expression of adhesion molecules, chemokines, and proinflammatory molecules. Regulatory T cells may also play an important role in the effectiveness of IVIg [116, 117].
IVIg is an alternative or adjunctive treatment for ANCA-associated vasculitis, with significantly less toxicity than standard therapy. A randomized clinical trial investigated the efficacy of IVIg (total dose 2 g/kg) in patients with recalcitrant Wegener’s granulomatosis and microscopy polyangittis. The study found partial or complete remission in 82 % of the IVIg group versus 35 % of the placebo group at 3 months. Despite these results, disease activity, frequency of relapse, and exposure to immunosuppression was the same in both groups, indicating that the benefit of IVIg was not maintained beyond 3 months [82, 98]. A prospective, open-label study of patients with Wegener’s granulomatosis and microscopic polyangiitis looked at the efficacy of adjunctive IVIg along with a standard immunosuppressive therapeutic for remission maintenance. At 9 months, 62 % were in complete remission, with 53 % maintaining remission at 24 months [118]. IVIg was used to successfully treat 12 patients with rapidly progressive glomerulonephritis from ANCA-positive vasculitis [91]. In another report, patients with Churg–Strauss syndrome were treated with IVIg in addition to corticosteroids, with or without cyclophosphamide [94]. In that study, motor neuropathy was improved in 13 of 15 patients and cardiac function improved in all five patients with heart failure.
In vitro studies have found IVIg inhibits ANCA induced neutrophil activation and cytokine release [119]. Using IVIg early in the course of ANCA-positive vasculitis may stop apoptotic cell death and reduce damage, particularly in serious forms such as Wegener’s granulomatosis [90]. Therefore, the use of IVIg may be justified as a first-line agent in this systemic vasculitis. It also offers an alternative to cytotoxic agents when contraindications exist. IVIg is usually administered for a total dose of 2 g/kg as a single dose or repeated in 4-week intervals. The patient response should be assessed after several intervals [120].
Kawasaki disease, also known as mucocutaneous lymph node syndrome, is an acute vasculitis of the small and medium sized vessels that typically presents in children between 6 months to 5 years of age. IVIg and high-dose aspirin together is considered the most appropriate and effective regimen for patients with this condition [121]. The standard treatment is 2 g/kg IVIg given as a single infusion within 10 days of symptom onset. Administration of IVIg after 10 days may alleviate symptoms, but it is not as effective in preventing coronary artery disease [122, 123]. In one study, 1 g/kg dose was just as effective [124]. Longer intervals may be used as well [125]. Although IVIg is the most effective treatment for reducing acute symptoms, 10–20 % of patients are resistant to initial IVIg treatment, putting them at risk for developing coronary artery disease [126]. Elevated IL-6, TNF-α, percentage of circulating neutrophils, and plasma clusterin are found in cases resistant to IVIg treatment [126–128]. Combination therapies may prove efficacious in resistant cases [129, 130].
Atopic Dermatitis
Atopic dermatitis (AD) is a common chronic inflammatory skin disease that usually starts in childhood. It is a complex genetically-determined disease often associated with other atopic disorders including asthma and allergic rhinoconjunctivitis [131]. Its most common features are pruritus and a chronic and relapsing dermatitis characterized by erythematous edematous papules/plaques, xerosis, and lichenification. Its exact etiology is unclear but thought to be due to an impaired epidermal barrier and dysfunctional stratum corneum that allows antigens to enter and activate immune cells.
A multi-faceted approach is needed to treat AD with the main focus being on avoiding triggers, a daily moisturizer to repair the skin barrier defect, topical steroids and topical calcineurin inhibitors, and education. Adjunctive modalities are used in severe refractory cases. These include systemic immunosuppressants (methotrexate, cyclosporine, oral corticosteroids, azathioprine, mycophenolate, etc.), phototherapy, IVIg, and targeted therapy with rituximab and omalizumab.
High dose IVIg, either as monotherapy or in conjunction with immunosuppression, has been reported to be effective in treating patients with recalcitrant disease, especially children [132–134]. However, there is a paucity of double-blind, placebo-controlled trials evaluating the use of IVIg for the treatment of AD. A report of 3 cases of adults treated with 2 g/kg monthly demonstrated improvement of skin lesions, reduction of concomitant systemic medications, and a decrease in serum IgE [135]. A randomized, placebo controlled study of 40 children with moderate to severe AD treated with three doses of 2 g/kg IVIg at 1 month intervals found that disease severity improved at 3 months [136]. A retrospective study of 10 children with severe refractory AD found similar results. Patients were treated with IVIg monthly for up to 24 months and found to have significant clinical improvement along with decreases in serum IgE. The clinical benefit varies with results lasting anywhere from 6 months to 4 years or longer [136–139]. On the other hand, a study of 10 patients evaluating the use of IVIg in adults with severe AD found that a single high-dose treatment did not significantly improve symptoms [140]. IVIg may provide benefit in AD patients by downregulating T cell function, particularily IL-4, and IL-5 and through its general immunomodulating effects [135]. Further randomized studies examining the effective dose, dosing intervals for initiation of therapy and maintenance are needed.
Mechanisms of Therapeutic Action of IVIg in Mucocutaneous Autoimmune and Inflammatory Diseases
The mechanism of action of IVIg is not completely understood but thought to be due to: neutralization of bacterial superantigens or other infectious agents, inhibition of TNF-α production, neutralization of pathogenic autoantibodies, regulation and modulation of Fc receptors, downregulation of proinflammatory cytokine production, suppression of the function of B lymphocytes, enhancement of regulatory T cells with inhibition of other T cells via T-cell receptor signaling, antioxidative effects, inhibition of TH17 differentiation, inhibition of differentiation of dendritic cells and suppression of the endocytosis of nucleosomes [69, 119, 121, 141, 142].
In immunobullous diseases, several mechanisms are thought to drive the decline in autoantibody levels and disease activity. A dramatic and rapid effect of treatment with IVIg is the selective decrease in autontibodies, leaving normal protective antibodies unaffected [23, 143, 144]. This correlates well with IVIg’s fast therapeutic effect. The rapid decrease suggests that this is due to increased catabolism of antibodies rather than decreased production [145]. A clinical study of 12 patients found that IVIg decreased anti-desmoglein 3 by 45 % and anti-desmoglein1 by 32 % from baseline within 2 weeks of the last cycle [146]. The lack of activity against long-term autoantibody production necessitates the use of cytotoxic immunosuppressants to supress production of new pathogenic autoantibodies during the catabolic phase [147].
The FcRn receptor is present on many cell types, inlcuding keratinocytes and endothelial cells. It functions to regulate serum immunoglobulin levels by protecting immunoglobulin from degradation. By increasing the concentration of IgG, these receptors become saturated, and are no longer able to protect IgG from degradation. This leads to increased catabolism of all antibodies. Because pathogenic antibodies are not replenished by IVIg therapy, levels of these antibodies decline, preventing their activation. In addition, IVIg may increase the expression of the inhibitory FcRIIB receptor on effector cells, resulting in decreased clearance of opsonized platelets [148]. This same mechanism could act to decrease activation of anti-keratinocyte primed phagocytes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree