CHAPTER 11 Intravascular Approaches to the Treatment of Varicose Veins
Radiofrequency and Lasers
The first attempt at minimizing the extent of surgery for varicose vein disease was the application of ligation alone. It was thought that the mere ligation of the saphenofemoral junction (SFJ) without disturbance of the great saphenous vein (GSV) with invasive techniques such as stripping the entire GSV to the ankle would be effective. Unfortunately, this minimal surgical treatment was demonstrated to result in a high degree of recurrence (upward of 50% at 3 to 5 years) even when the ligation was accompanied by sclerotherapy or ambulatory phlebectomy of distal varicose veins.1–5 When these recurrences where evaluated, treatment failure was secondary to reanastomosis through hemodynamically significant perforator or anastomotic veins extending from the knee to the groin, which remained in place after ligation alone.6 Since ligation alone failed to provide acceptable degrees of improvement in abnormal venous hemodynamics, it was recommended that more invasive complete removal of the GSV from the SFJ to the knee after ligating the SFJ be performed. However, stripping typically required general anesthesia, with patients usually taking a week or more to get back to normal activities. So it appeared that, due to lack of effectiveness, the first attempts at reducing the extent of surgery by ligation alone failed to gain acceptance. Ironically, the continued necessity for stripping probably spurred the development of endovenous techniques as many patients would shudder at the thought of stripping. The race was on to develop a minimally invasive alternative using intravascular laser and RF devices to thermocoagulate endothelial cells and vein walls, producing specific destruction of the targeted vessel without the necessity of stripping or ligation.
Radiofrequency Closure
The first theories of endovenous ablation were based on the belief that specifically directing relatively omnidirectional RF energy into vein walls to cause their destruction was potentially safer, easier to engineer and more controllable than other mechanisms for doing so. Initial designs involved a mechanism by which RF current heated tissue by resistive (or ohmic) heating of a narrow rim (less than 1 mm) of tissue in direct contact with an electrode. Deeper tissue planes could be slowly heated by conduction from the small-volume region of heating, although heat was typically dissipated by conduction into surrounding normothermic tissue.7 By carefully regulating the degree of heating with microprocessor control, subtle gradations of either controlled collagen contraction or total thermocoagulation of the vein wall could be achieved.
The initial design was such that when the RF catheter was pulled back, a feedback-controlled loop regulated by readings from a thermocouple enabled the operator to heat a section of vein wall to a specified preset temperature. This was chosen for its relative safety since the temperature increase remained localized around the active electrode. This necessitated the maintenance of close, stable contact between the active electrode and the vessel wall without coagulum formation. It was believed by strictly limiting the temperature to 85°C, boiling, vaporization, and carbonization of the tissues could be avoided.8 It was also believed that heating the endothelial wall to 85°C resulted in heating the vein media to no more than 65°C, the minimal temperature at which collagen contracts.
Ex-vivo studies by Reich-Schupke et al9 investigated histological changes following radiofrequency ablation at various powers and application times. When low power (5 W) and an application time up to 400 ohms was applied, histological changes were not uniform. Necrosis was limited to the endothelium in the majority of vein segments and rarely reached the media. This would most likely not result in complete vein shrinkage and occlusion. At 20 W and an application time up to an impedance of 400 ohms, histological changes included widespread necrosis of the initima and media and collagen bundle coagulation. The authors concluded that with increased power and application time, there was a more homogenous and extensive heating of the vein wall, which was thought to lead to a more successful outcome.
Vessel wall ablation using electrode-mediated RF is a self-limiting process. As coagulation of tissue occurs, there is a marked decrease in impedance that limits heat generation.10 Alternatively, if a clot builds up on the electrodes, blood is heated instead of tissue and there is a marked rise in impedance (resistance to RF). The RF generator can be programmed to rapidly shut down when impedance rises, thus assuring minimal heating of blood but efficient heating of the vein wall. The problem is that the electrodes must be manually debrided of coagulum, which requires the removal of the catheter, cleaning by the operator and then reinsertion – which is problematic during tumescent anesthesia.
The initial experience, dating back to 1998, demonstrated an efficacy equal to or better than that of ligation and stripping, with few, if any, adverse sequelae.11–23 Early experience directly comparing RF Closure with ligation and stripping procedures, even with RF performed under general anesthesia, noted equal efficacy with less pain, shorter ‘sick leave’, and faster return to normal activities.18
When performed by us, the procedure was entirely under local tumescent anesthesia, with over 90% of patients resuming normal activities 1 to 2 days postoperatively. Its main drawbacks were the high cost of single-use catheters and the necessity to withdraw the catheter manually at a speed of 2 to 3 cm per minute and frequent cleaning of coagulum on the electrodes, which made the procedure tedious at times. To speed up the procedure, Goldman12 recommended that only the most proximal 20 cm of the GSV be treated with RF and the remaining varicose GSV be treated with ambulatory phlebectomy, but this technique has not found wide acceptance. Goldman believes that the addition of ambulatory phlebectomy minimizes the possibility of recurrence from distal perforators. Proebstle et al24 found that up to 30% of tributary veins do not resolve with laser ablation of the GSV alone, thereby necessitating removal with ambulatory phlebectomy.
However, treatment of the GSV or its tributaries below the knee may not be entirely necessary, as others have shown that ligation and stripping procedures from the groin to the knee add little to the procedure’s efficacy.15,25 In addition, others have also demonstrated equal effectiveness with less than 2 years follow-up when only the proximal 30 to 40 cm of the GSV is treated without treating distal varicose tributaries.13–15,17,26
Weiss and Weiss17 evaluated patients treated with a percutaneous approach allowing access of the Closure catheter to treat the proximal GSV. Patients (mean age, 47.2 ± 12.6 years; 76% female) had symptomatic saphenous reflux with a saphenous vein diameter of 2 to 12 mm (mean, 7.4 mm). Most of the veins treated were above-knee great saphenous (73%), some entire great saphenous (21%), with the remaining including below-knee great saphenous, small saphenous, and accessory saphenous. Adjunctive procedures performed at the time of treatment were phlebectomy on more distal branches in 61% and high ligation in 21%, but the adjunctive procedures did not affect outcome.
Vein occlusion at 1 week was documented by duplex ultrasound in 300 out of 308 legs, or a success rate of 97%. Occlusion persisted at 6 weeks in 95% and at 6 months in 92%. In this report, if the saphenous vein was closed at 6 months it was noted by duplex ultrasound to remain closed to 12 months and beyond. Subsequent follow-up for up to a decade by duplex ultrasound indicates that any vein noted to have been eliminated at 12 months by RF will never recur. Typically when the GSV is treated there is closure or elimination of major tributaries at the SFJ except for the superficial or superior epigastric vein, which, intentionally not treated, continues to empty superiorly into the common femoral vein. We believe that there is a high margin of safety by maintaining flow through this tributary. The high flow rate appears to diminish the possibility of extension of any thrombus (in the unlikely event that this would occur) from the GSV and has the additional benefit of allowing normal venous flow from the lower abdominal wall into its proper drainage into the common femoral vein. By leaving the superior epigastric vein intact, thrombus in the GSV following this procedure has not been observed.13
Long-term efficacy with the RF ablation has been documented by Merchant et al27 investigating 1222 limbs (great saphenous, small saphenous, and accessory saphenous veins). Occlusion rates (evaluated via duplex ultrasound) of 96.8%, 89.2%, 87.1%, 88.2%, 83.5%, 84.9%, and 87.2% were found at 1 week, 6 months, 1 year, 2 years, 3 years, 4 years, and 5 years, respectively. Body mass index greater than 25 was associated with an increased incidence of nonocclusion, groin reflux, and recanalization. A pullback speed above 3 cm/minute at 85°C was more likely to result in nonocclusion and recanalization. In the study by Vasquez et al,28 factors associated with improved occlusion rates included increasing age, female sex, and volumes greater than 250 ml of tumescent anesthesia. The authors theorized that increased failure rates associated with male sex and younger age are secondary to variations in collagen and inflammation in these populations.
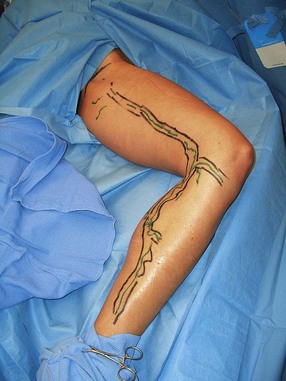
In a study by Goldman and Amiry,16 closure of the GSV with endoluminal RF thermal heating in combination with ambulatory phlebectomy was equally as effective as closure of the GSV as described above. The first 47 sequential, nonrandomized patients having an incompetent GSV from an incompetent SFJ and painful varicosities in 50 legs were treated with the VNUS Closure procedure. The varicose veins were marked with the patient standing and again with the patient lying down in the operative position with a venoscope (LLC, Lafayette, La.), as previously described (Fig. 11.1).12,29,30 After appropriate marking, the area surrounding the GSV and distal tributaries to be treated was infiltrated with 0.1% lidocaine tumescent anesthesia. The amount of tumescent fluid averaged 800 mL with a lidocaine dose of 8 mg/kg. The GSV was then accessed through a 2- to 3-mm incision in the medial midthigh, usually 20 cm inferior to the SFJ. The proximal portion of the GSV was then treated with VNUS Closure and the distal portion, including all varicose tributaries, was removed with a standard ambulatory phlebectomy technique.
Three separate papers detail a similar cohort of patients treated in multicenter studies encompassing from 16 to 31 clinics, 210 to 324 patients, and 6 to 12 month follow-up.13,14,31 The vein occlusion rate at 1 year examination was 91.6% from nine centers and 81.9% from fourteen centers. Forty-nine patients were followed at 2 years with duplex scans and showed an 89.8% closure rate. There was a 3% incidence of paresthesia which was decreased to 1.6% when treatment was confined to the thigh. Two limbs (0.8%) developed scarring from skin burns and three patients developed a deep vein thrombosis (DVT) with one embolism. The reason for the increase in adverse effects appears to be the use of general anesthesia without tumescent anesthesia by a majority of the surgeons.
Sybrandy and Wittens32 from Rotterdam reported one year follow-up of 26 patients treated with VNUS Closure. They reported five patients with postoperative paresthesia of the saphenous nerve and one with a cutaneous burn, for an overall complication rate of 23%. One patient (3.8%) had total recurrence of the GSV. One patient (3.8%) could not be treated due to a technical failure. Eight patients (30.8%) had closure of the GSV, but with persistent reflux of the SFJ. Thirteen patients (50%) had closure of both the GSV and SFJ. Overall, 88% of patients had a totally occluded GSV.
Another report describes two episodes of DVT in 29 patients treated with the RF Closure.33 Here, the surgeons treated the patient with a groin incision and passage of the catheter from the groin downward. The authors do not report the type of anesthesia used or the length of vein treated. It is presumed that patients were not ambulatory and were treated under general anesthesia.
In our experience using tumescent anesthesia in awake patients, two patients have developed focal numbness 4 cm in diameter on the lower medial leg. These resolved within 6 months. Since adopting the principles outlined above of tumescent anesthesia and moving the catheter rapidly from any points of sharp pain, no paresthesias have been noted. No skin injury or thrombus has been observed in any of our patients. Unfortunately, with both endoluminal RF and laser procedures, if patients are not ambulatory after the procedure and/or if tumescent anesthesia is not given, complications in the form of DVT, PE, or angiogenesis have been reported.34–36 Tumescent anesthesia or the placement of large volumes of dilute anesthesia in a perivascular position serves several purposes:
Contrary to the report by Hingorani et al34 we have never seen DVT in any of our patients treated with intravascular laser or RF. We believe that the reason for our lack of adverse sequelae is the use of tumescent anesthesia in awake patients with immediate ambulation and avoidance of occlusion of the superior epigastric vein. While we realize treating patients without general anesthesia is not standard practice for general or vascular surgeons,37 some vascular surgeons who perform tumescent anesthesia on awake patients with immediate ambulation have reported similar results with virtually no DVT. A DVT was noted in a female patient treated using tumescent anesthesia while awake but she weighed more than 350 pounds and did not ambulate after the endoluminal RF procedure.38,39
Salles-Cunha et al35 reported on the development of angiogenesis and fibrotic tissue along the course of the GSV treated with RF Closure. Contrary to this report, our experience with tumescent anesthesia utilized is a complete lack of detection of small vessel networks (angiogenesis) by duplex ultrasound. We believe that the reason for our lack in detecting small vessel networks is not from a lack of trying to see them, but from the minimization of inflammation that occurs with tumescent anesthesia placed in the perivascular space during either RF or laser endothelial ablation.40
ClosureFAST
In 2006, VNUS introduced the ClosureFAST catheter. This new device promised increased time efficiency and ablation of incompetent veins of any size. The 7F ClosureFAST catheter allows 7 cm segments of vein to be uniformly heated for 20 seconds at 120°C. The temperature is maintained by a radiofrequency generator through a feedback loop, and vein segments are treated serially41 with continuous pullback not needed.42 While treatment with the Closure system was limited to veins of less than 12 mm, no diameter restrictions are indicated with the ClosureFAST catheter.41,42 The manufacturer recommends the initial and most proximal 7 cm of great saphenous vein to be treated with two consecutive cycles, while the remaining vein segments may be treated with a single cycle. Each disposable catheter is US$795 (as of 11/2009). Proebstle et al41 treated, 252 GSVs with ClosureFAST and either adjuvant ambulatory plebectomy (in 71.6%) or foam sclerotherapy (in 13.9%). Mean treatment time (spanning the time between catheter insertion and removal) was 16.4 ± 8.2 minutes and 6.7 ± 1.7 treatment cycles. The linear endovenous energy density was 116.2 ± 11.6 J/cm for the initial 7 cm of GSV, and 68.2 ± 17.5 J/cm for the subsequent 7 cm. Patients were followed at 3 days, 3 weeks, 3 months, and 6 months post procedure. All patients had successful occlusion of their GSV. Via life-table analysis, occlusion rates were 99.6%. Seventy percent of patients experienced no postprocedural pain. No deep vein thrombosis or skin burns were seen. Side effects were infrequent with 3.2% paresthesias, 0.8% phlebitis, 1.6% hematomas, 2% hyperpigmentation, and ecchymoses in 6.4%. Mean patient down time was 1.0 ± 1.9 days. Finally, 99% of treated patients would recommend the ClosureFAST system to their friends.
Calcagno et al43 investigated the relationship of size to efficacy in 338 great and small saphenous veins following ClosureFAST treatment. Initial occlusion rates, evaluated between postoperative days 2 to 5, were not significant (94% in veins ≤ 12 mm and 96% in those > 12 mm). At 6 months, complete occlusion rates in veins ≤ 12 mm or > 12 mm were similar (98% and 100%, respectively). Interestingly, veins partially occluded in the immediate postoperative period developed complete occlusion at 6 months follow-up. Diameter did not affect the outcome for successful treatment of incompetent saphenous veins with ClosureFAST.
The Recovery Study by Almeida et al,44 compared 87 GSVs treated with either ClosureFAST or 980-nm diode endovenous laser. This small, short term follow-up study of only 1 month, demonstrated increased incidence of eccyhmoses, pain, phlebitis, and tenderness in the 980-nm laser group during the initial postoperative 2 weeks. These increased side effects were attributed to microperforations caused by the 980-nm diode. While quality of life and venous severity scores were more favorable in the initial 2 weeks in the ClosureFAST group, no difference was seen at 1 month follow-up. No comparisons of efficacy were provided in this short-term study.
Long-term studies are necessary to assess prolonged efficacy of the ClosureFAST system. Radiofrequency and 1320-nm Nd:YAG laser both stimulate collagen contraction, have negligible development of thrombi, and show decreased incidence of side effects, owing to lack of perforations of the vein wall.45 We feel a randomized, blinded trial comparing the efficacy and safety of these two technologies is justified.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree