Fig. 42.1
(a) Preoperative patient with grade 3 abdominal deformity on the Pittsburgh scale. (b) Three months after anchor abdominoplasty
During the study, they received daily nutritional supplement (Materna®, Wyeth, São Paulo, Brazil) containing 60 mg of iron, 1 mg of folic acid, and 12 μg of vitamin B12, among other ingredients. No blood transfusion was performed as 7 g/dL was defined as the cutting point for indication. Twenty patients were in a treatment group and 12 patients were in a control group. Treatment group had anchor abdominoplasty as described by Costa et al. [26]. Surgical planning was done by bimanual palpation to estimate the amount of excessive tissue in the middle vertical zone and suprapubic zone.
Surgical procedures were done under general anesthesia, urinary catheterization, and intermittent pneumatic compression in the lower limbs. Surgery began with resection of the vertical component, followed by the horizontal component with a scalpel on the skin and electrocautery in the other tissue layers. Surgical samples were weighted and abdominal diastasis was treated. Vacuum suction drains were applied before suturing in a three-plane fashion. During all the time in the operating room, patients received maintenance intravenous saline solution during whose volume was calculated by multiplying the subject’s weight in kilograms by 1.5 mL.
Patients received maintenance intravenous saline solution for 12 postoperative hours and had urinary catheterization for 24 h. They were discharged from hospital on second postoperative day with prescription of antibiotics, pain killers, and anti-inflammatory drugs for 1 week. Suction drains were removed when daily volume of drainage was less than 50 mL.
Treatment group had blood tests on the evening prior to surgery and 7, 28, and 56 days after surgery. Hemoglobin was also tested at 48 postoperative hours. Control group had blood tests scheduled in the day of first consultation and 7, 28, and 56 days after the first collection. Blood tests were hemoglobin, reticulocytes, serum iron, transferrin, transferrin saturation, and ferritin. All data was submitted to statistical analysis.
No difference in hematological variables or iron status was identified between control and treatment group before abdominoplasty. Hemoglobin had a decrease in 2 g/dL on the second postoperative day. In 1 week, one-third of this deficit was recovered, but until the eighth week, hemoglobin levels did not come up to preoperative status. Absolute reticulocyte count significantly increased during the first postoperative week and returned to preoperative levels in 4 weeks. In control group, no change was detected in reticulocyte count or iron profile during the 8-week follow-up (Fig. 42.2).
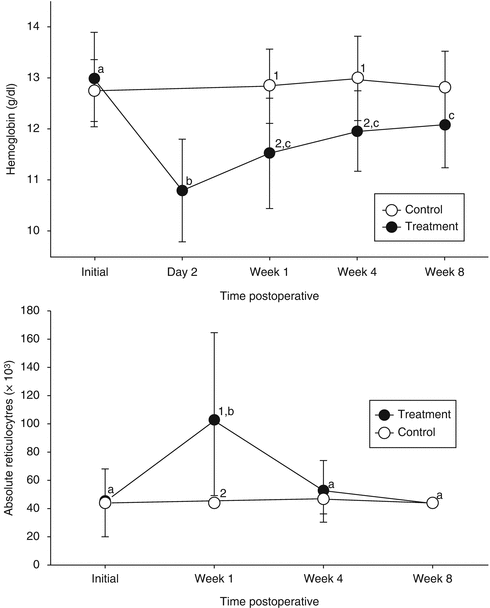

Fig. 42.2
Distribution of hemoglobin levels (g/dL; top graph) and absolute reticulocyte count (μL; bottom graph) in the treatment (black circle) and control (white circle) groups over time. Values with different superscripts within each group over time (a, b, c) or between groups at each time points (1, 2) were different (p < 0.05)
In treatment group, serum iron had a decrease of about 34 μg/dL (from 88 to 54 μg/dL), and it remained below preoperative levels until 8 weeks. Transferrin decreased during the first week but returned to preoperative levels by the end of the study follow-up. The transferrin saturation index (TSI) presented the same behavior as transferrin. Ferritin increased during the first week after surgery and decreased during the following weeks reaching the eighth week with levels below preoperative ones.
At the end of the study, nine patients from the treatment group had ferritin levels under 11 ng/mL, indicating an iron deficiency, and 11 patients had ferritin levels above that. Patients with ferritin <11 ng/mL in the eighth week had a hemoglobin deficit statistically higher than what was found in patients with ferritin >11 ng/mL (Fig. 42.3).
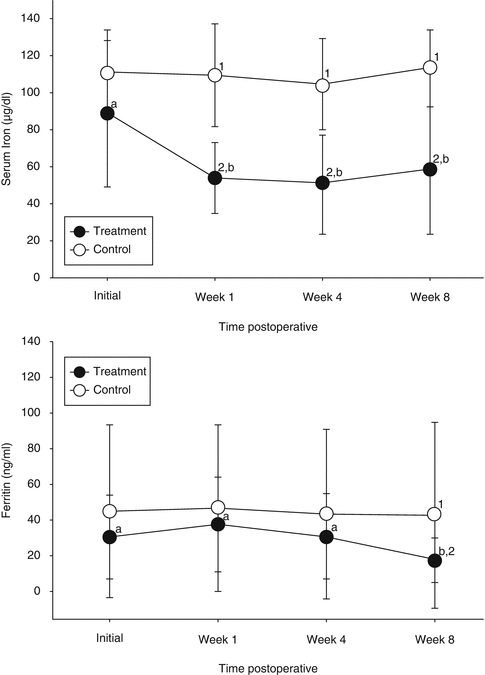

Fig. 42.3
Concentrations of serum iron value (μL; top graph) and ferritin (ng/mL; bottom graph) in the treatment (black circle) and control (white circle) groups over time. Values with different superscripts within each group over time (a, b, c) or between groups at each time points (1, 2) were different (p < 0.05)
42.3 Discussion
Reduction in iron ingestion, gastric acid secretion, and contact of food with absorptive mucosa are the main mechanisms involved in iron deficiency after bariatric surgery and can cause a reduction of 40.3 % in iron absorption in the first 6 months after surgery [27]. In order to prevent anemia and promote a better iron absorption, multivitamin supplements are regularly prescribed for patients [10]. However, most of commercial multivitamin complexes contain less iron than they actually need. Current recommendations for iron intake in postbariatric patients are of 40–65 mg/day of elemental iron and might be a little higher for women in reproductive age due to menstrual bleeding [28]. This level of elemental iron is present in only a few supplements for prenatal use. A double-blind randomized clinical trial showed that oral administration of 320 mg of iron sulfate twice a day prevented iron deficiency [29]. Whenever oral iron supplements are not enough for preventing deficiency, patients should be prescribed parenteral (intravenous, mainly) administration [11].
When studying iron profile, it’s important to remember that it can be changed by many factors other than iron absorption. Other micronutrient deficiencies, such as vitamin B12, folic acid, and albumin can influence changes in transferrin level, as it is a hepatic protein associated to malnutrition [30]. Inflammatory status identified by high protein C levels can also affect iron profile by increasing ferritin levels [31]. Dehydration, smoking, pregnancy, increased blood capacitance, crystalloid boluses, and even laboratory errors are often able to cause changes in hemoglobin levels.
Surgical technique should be accurate to minimize blood loss and time in the operating room. Intraoperative blood loss is not well estimated by weighting sponges or measuring contents of suction machines. It can be indirectly estimated by hemoglobin levels on the second postoperative day, which are clinically more relevant as well [32]. Resection of excessive skin and fat may lead to a decrease of 2 g/dL in anchor abdominoplasty [24] or 3 g/dL in circumferential abdominoplasty [33]. Maintenance saline solution does not result in hemodilution [34]. When given in bolus, saline is able to reduce hemoglobin level for 8 h with total recovery after that.
At the end of the surgery, the organism starts to recover from surgical trauma. Erythropoietin-stimulated bone marrow can increase its cell production capacity severalfold causing a medullar erythroid hyperplasia which is noticeable after 3–5 days [35]. Hemoglobin levels increased significantly at seven postoperative days, correcting one-third of the hemoglobin deficit due to an increase in erythopoiesis. However, after a rapid recovery in hemoglobin levels during the first week, there were no significant increases in the fourth and eighth weeks. At the end of the study, the hemoglobin levels remained significantly lower than preoperative levels [24].
The postoperative recovery from anemia is not well understood yet. Most of the studies about it regard hip arthroplasty in elderly patients [36–38]. They found an incomplete recovery of hemoglobin levels, reduced serum iron and transferrin levels, and increased ferritin levels in an eight-week follow-up. Those findings are also present in chronic disease anemia profile and have an inflammatory pathophysiology. The key phenomenon in this kind of anemia is the inability of iron stored in the reticuloendothelial system to be released to bone marrow for erythropoiesis [39, 40]. Cytokines such as interleukin-6 (IL-6) and C-reactive protein are involved in postoperative acute-phase inflammation and are increased after surgery [37]. IL-6, one of the most important mediators of this process, is responsible for an increase in hepcidin levels. Hepcidin is a liver hormone that reduces iron absorption by duodenal mucosa and release of iron from reticuloendothelial system promoting low serum iron levels [41, 42]. It is clear, then, that inflammatory response to surgical trauma limits erythopoiesis in postbariatric patients as well [24, 37].
However, as iron is essential to erythropoiesis, reduced levels of iron may also be responsible for postoperative anemia [36]. If patients have previous low storage of iron expressed by low ferritin levels preoperatively or develop iron deficiency with low ferritin 6 weeks after surgery, they are prone not to recover hemoglobin levels as they cannot sustain an intensified erythropoiesis as needed to compensate intraoperative blood loss [24, 36]. Because of the tendency for iron deficiency, postbariatric patients have low ferritin levels preoperatively (ferritin levels <30 ng/mL) [28]. What is the minimum preoperative ferritin level necessary to maintain adequate erythropoiesis after postbariatric abdominoplasty without depleting iron reserves? A theoretical calculation revealed that preoperative ferritin should be around 56 ng/mL to enable correction of the average deficit of 2.18 g/dL hemoglobin found in postbariatric abdominoplasty without depleting iron reserves [24].
This study suggests that intravenous supplementation with iron during the preoperative or perioperative period may be indicated for candidates of postbariatric abdominoplasty, to avoid postoperative depletion of iron reserves and to accelerate postoperative recovery of hemoglobin levels. Randomized and controlled prospective trials are under way to test this proposal (ISAPA trial, NCT01857011). In postbariatric patients with preoperative low ferritin levels, intravenous iron supplementation perioperatively might be a good strategy as it has been demonstrated for orthopedic patients [43]. Thus, ideal conditions would be provided for these patients for postoperative recovery of hemoglobin levels without depleting iron reserves, and next plastic surgeries would safely be kept on schedule.
42.4 Conclusions
Postbariatric patient candidates to plastic surgery should have a comprehensive preoperative evaluation as they have a higher risk of iron deficiency. Not only hemoglobin levels but also iron profile, particularly ferritin levels, must be known [44




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







