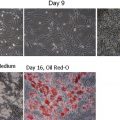
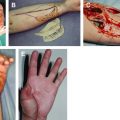
Cutaneous wound healing is a dynamic process with the ultimate goal of restoring skin integrity. On injury to the skin, inflammatory cells, endothelial cells, fibroblasts, and keratinocytes undergo changes in gene expression and phenotype, leading to cell proliferation, migration, and differentiation. Cytokines and growth factors play an essential role in initiating and directing the phases of wound healing. These signaling peptides are produced by a variety of cells and lead to a concerted effort to restore the skin barrier function.
- •
The Food and Drug Administration has approved the use of recombinant platelet-derived growth factor for the treatment of lower extremity diabetic neuropathic ulcers that extend into the subcutaneous tissue or beyond and have adequate blood supply.
- •
Fibroblast growth factor 2, granulocyte-macrophage colony-stimulating factor, and vascular endothelial growth factor are the most promising cytokines and growth factors for the enhancement of wound healing.
- •
Debridement is the most important step in the treatment of chronic wounds.
Introduction
The body’s response to skin injury is focused on :
- 1.
Rapid wound closure
- 2.
Restraining invasion of microorganisms
- 3.
Preventing excessive fluid loss.
The early response is activated immediately after injury, resulting in the inflammatory phase (first stage) of wound healing. After hemostasis a fibrin clot is formed, which later serves as a scaffold for infiltrating cells. In addition, neutrophils and monocytes are recruited to the wound in response to trauma and bacterial contamination.
The second stage of wound repair occurs approximately 2 to 10 days after the injury and is characterized by proliferation and migration of different cell types. Keratinocytes migrate over the wound bed while fibroblasts and macrophages replace the fibrin clot with granulation tissue. The newly formed immature dermis is neovascularized, and the keratinocytes behind the leading edge proliferate and differentiate to restore the barrier function of the epidermis.
Tissue remodeling, the third stage of wound repair, begins 2 to 3 weeks after injury and lasts for 1 year or more. The type III collagen that is deposited in the initial stages of wound healing is slowly replaced by type I collagen, thereby forming the mature dermis.
An increased understanding of the molecular mechanisms that regulate the various events of wound healing has laid the foundation for therapeutic interventions attempting to improve the healing outcome. The cell-cell and cell-matrix interactions are fundamental for successful wound healing, and growth factors and cytokines maintain the balance of signals that regulate cellular migration, proliferation, and adhesion to a large extent. Malfunction leads to a prolonged healing time or complete failure to heal and may result in a chronic wound. The wound fluid from chronic wounds has an increased concentration of proinflammatory cytokines in comparison with wound fluid from acute wounds. By contrast, there is a decreased concentration of growth factors in chronic wounds with high protease activity and decreased levels of natural protease inhibitors. This deficiency in growth factors can be attributable to decreased production or secretion, more rapid breakdown, and, as is the case in venous stasis ulcers, binding to macromolecules, making them nonfunctional.
Cytokines and growth factors in wound healing
Cytokines in Wound Healing
Cytokines are peptides and glycoproteins with a molecular weight of 5 to 30 kDa and are primarily produced by inflammatory cells. Cytokines regulate inflammatory and immune responses during wound healing by activating various cells. The cytokines include chemokines, lymphokines, monokines, interleukins, colony-stimulating factors, and interferons, and can be distinguished from growth factors by the type of cells they influence. Inflammatory cytokines are believed to have roles in wound healing, including migration and proliferation of keratinocytes and fibroblasts. Interleukins 1 and 6 (IL-1 and IL-6) and tumor necrosis factor α (TNF-α) are upregulated during the inflammatory phase of wound healing and are important in modulating reepithelialization. The granulocyte-macrophage colony-stimulating factor (GM-CSF) is involved in the activation of neutrophils and macrophages, and alters the activity of keratinocytes and fibroblasts.
Interleukins in Wound Healing
In an acute wound, IL-1 is produced by monocytes, macrophages, fibroblasts, and keratinocytes. Besides serving as a paracrine factor, IL-1 acts as an autocrine signal that induces the migration and proliferation of keratinocytes. Exogenously administered IL-1 has been shown to promote healing of partial-thickness wounds in swine. In a prospective, double-blind, placebo-controlled trial, various concentrations of IL-1β were administered to patients with pressure ulcers daily for 28 days. No significant differences were noted in any of the treatment groups.
TNF-α in Wound Healing
Similar to IL-1, TNF-α is a proinflammatory cytokine that is produced by many cell types during wound healing. The TNF-α level is elevated in chronic wounds, and its expression diminishes as healing progresses. In a study by Streit and colleagues, the TNF-α antagonist infliximab was administered daily to 14 leg ulcers. After 4 weeks of treatment, the surface area of the ulcer was reduced by more than 50% in 6 of the 14 leg ulcers.
GM-CSF
GM-CSF is one of the most widely studied cytokines in wound healing. Besides being a potent activator of neutrophils and macrophages, GM-CSF influences the activity of keratinocytes and fibroblasts and increases the production of vascular endothelial growth factor (VEGF). In a double-blind, randomized, controlled study of 60 patients with venous stasis ulcers, GM-CSF was perilesionally injected weekly for 4 weeks. Administration of GM-CSF significantly improved the rate of healing by approximately 60%.
Growth Factors in Wound Healing
Growth factors are regulatory peptides that are synthesized and secreted by many of the cell types involved in wound healing, including inflammatory cells, platelets, fibroblasts, epithelial cells, and endothelial cells. Growth factors cause cells to migrate by chemotaxis, proliferate, differentiate, and synthesize extracellular matrix components. Growth factors may act by autocrine, paracrine, juxtacrine, or endocrine signaling. On binding to the cell receptors, the growth factors trigger a cascade of intracellular events, leading to the activation of transcription factors, which results in gene expression.
Growth factors are classified into several families based on their characteristics. The most relevant growth factor families for wound healing are the epidermal growth factor (EGF), fibroblast growth factor (FGF), transforming growth factor β (TGF-β), platelet-derived growth factor (PDGF), and VEGF. The different growth factor families are summarized in Table 1 .
| Cells | Effects During Wound Healing | |
|---|---|---|
| Cytokine | ||
| IL-1 | Neutrophils Monocytes Macrophages Keratinocytes | Inflammation, reepithelialization, increased levels in the acute and chronic wound |
| Growth Factors | ||
| EGF | Platelets Macrophages Fibroblasts | Cell motility and proliferation, increased levels in the acute wound, decreased levels in the chronic wound |
| TGF-β1 and TGF-β2 | Platelets Keratinocytes Macrophages Lymphocytes Fibroblasts | Reepithelialization and inflammation, granulation tissue formation, fibrosis and tensile strength, increased levels in the acute wound, decreased levels in the chronic wound |
| PDGF | Platelets Keratinocytes Macrophages Endothelial cells Fibroblasts | Chemotaxis, inflammation, granulation tissue formation, matrix remodeling, increased levels in the acute wound, decreased levels in the chronic wound |
| VEGF | Platelets Neutrophils Macrophages Endothelial cells Fibroblasts | Angiogenesis, granulation tissue formation, increased levels in the acute wound, decreased levels in the chronic wound |
| IL-1 | Neutrophils Monocytes Macrophages Keratinocytes | Inflammation, reepithelialization, increased levels in the acute and chronic wound |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree