22 Hands and feet
Summary and Key Features
• Extrinsic and intrinsic factors cause skin laxity, dyspigmentation and fat loss in the hands.
• Soft tissue augmentation with a variety of fillers has been well studied and shown to be safe and efficacious to camoflouge exposed tendons and vessels and improve lipoatrophy of the hands.
• The most common fillers for the hands are calcium hydroxyapatite, hyaluronic acid and autologous fat.
• Poly-L-lactic acid has fallen out of favor for hand augmentation because of the risk of visible nodules under lax skin.
• Use of disposable blunt tipped cannulas has improved the ease and reduced the side effect profile for hand filling.
• In addition to standard post-filler instructions like ice and elevation, patient should be told to massage the hand after treatment.
• Potential complications of hand augmentation include visible product, Tyndall effect, persistent edema and pain.
• Dermal fillers have been used to improve symptoms and speed healing of diabetic foot ulcers. The use to reduce metatarsalgia symptoms from chronic wearing of high heels is being used but has not been well studied.
Soft tissue augmentation of the hands
Calcium hydroxylapatite
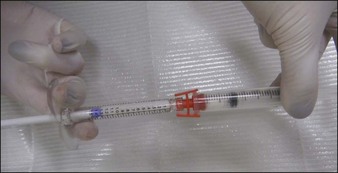
As an opaque, white substance, CaHA has the advantage of acting as both a filler and camouflage for underlying anatomical architecture. However, its use for hand rejuvenation was initially limited by the product’s high viscosity and pain upon injection. In 2007, Busso & Applebaum reported a novel approach that overcame both issues. Utilizing a Luer-to-Luer lock connector (Fig. 22.1A), the authors mixed 0.1 mL 2% lidocaine without epinephrine with 1.3 mL of CaHA (the standard syringe volume at that time). After tenting the skin of the central dorsal hand to separate it from underlying large vessels and tendons, they injected a single bolus of 0.5–1.4 mL of the lidocaine–CaHA mixture into the space created between the subcutaneous layer and the superficial fascia. The bolus was then massaged and molded into a cosmetically smooth appearance. The addition of lidocaine reduced both the viscosity and pain.
Several larger studies by Busso et al, Bank, and Marmur et al have recently been published supporting the safety and efficacy of CaHA as an anti-aging hand filler. Busso et al published a multicenter, randomized, controlled trial of 101 patients followed over 6 months and established a new hand volume severity scale 3 to assess results (Table 22.1). Following administration of a lidocaine bolus preoperatively, CaHA was administered as a bolus into the dorsal hand using a 27-gauge 0.75-inch (20 mm) needle. Aside from reporting statistically significant improvement in the treatment group, the study also found that adverse events, including bruising, itching, pain, redness, and swelling (Fig. 22.2), were frequent yet short in duration and did not affect overall patient function significantly. Similarly, Sadick noted only brief and minor side effects in 10 patients following CaHA treatment with a 25-gauge 0.5-inch (12 mm) needle to the dorsal hands and residual cosmetic correction lasting up to 1 year postoperatively.
Table 22.1 Busso hand volumizing severity scale
| Severity | Characteristics of hand |
|---|---|
| 4 | All three central tendons are fully exposed when hand is at rest |
| 3 | All three central tendons are partially exposed, with one or two tendons fully exposed when hand is at rest |
| 2 | All three central tendons are partially exposed when hand is at rest |
| 1 | One or two central tendons are slightly exposed when hand is at rest |
| 0 | No tendon is exposed when hand is at rest |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree