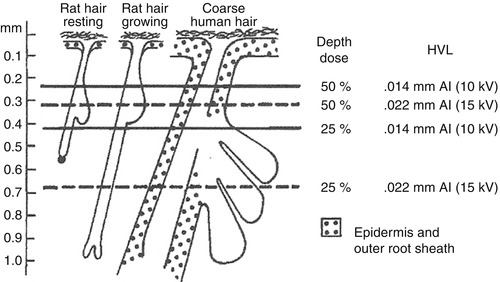
Fig. 5.1
Absorption curves (From Ebbehoj)
It appears that the biological effect of grenz ray is localised to the absorbed area – clinical benefits in treating dermatoses are limited strictly to the irradiated area.
The exact mechanism of action of grenz rays is unknown.
It appears to exert its effect by affecting the afferent arm of the immune response.
5.3.1 Effects on Langerhans Cells
There have been a number of studies showing effects on Langerhans cells in the epidermis [1, 30]. Following 4 G 10 kV grenz ray, Langerhans cells were reduced significantly at 1 and 3 weeks after irradiation. Comparing 3 × 4 G grenz ray weekly to 3 × 30 J/cm2 UVA (suberythemal) weekly, there was a marked decrease in epidermal Langerhans cells in grenz ray-treated sites and those that remained showed little change (fewer Langerhans cell granules) – keratinocytes and intercellular spaces were unaffected. No Langerhans cells were found in the dermis in grenz ray-treated nor control skin. By contrast low-dose UVA did not show reductions in Langerhans cells in the epidermis (high-dose UVA, low-dose UVB and PUVA do). The Langerhans cells showed an increase in Langerhans cell granules, mitochondria and enlarged Golgi apparatus. A few sunburn cells were seen but keratinocytes in general appeared unaffected by low-dose UVA.
The fate of the Langerhans cells removed from the epidermis has not been determined. As the Langerhans cells return, it is speculated that the Langerhans cells probably migrate to the draining lymph nodes as part of the afferent arm of the local immune response.
5.3.2 Effects on Dermatitis
By pretreating nickel allergic patients with grenz rays (3 × 3 G weekly) then applying nickel patch tests on treated and untreated skin, it is has been shown possible to significantly reduce allergic contact dermatitis reaction. This reduction lasts 3 weeks and correlates with reduction in the epidermal Langerhans cell population.
There was a tendency towards weaker irritant reactions with sodium lauryl sulphate pretreating patients with grenz ray although this was not statistically significant [29]. Grenz rays reduced itch but not flare following intradermal injection of histamine, but this was not statistically different from placebo [8]. Grenz rays can decrease histamine levels and mast cells in rat skin (although control animals also showed similar changes) [3]. Grenz ray can stimulate amino acid production in the epidermis similar to tape stripping.
5.3.3 Factors Affecting Grenz Ray Erythema
Grenz ray erythema can be inhibited by a single application of hydrocortisone ointment applied 6 h prior and washed off 1 h before irradiation [19], but concomitant therapy with grenz ray and topical corticosteroids for psoriasis did show an additive effect in scalp psoriasis [28].
Bergamot oil application can encourage development of erythema in grenz ray fields [36].
5.3.4 Regional Skin Sensitivity Variability
Kalz [18] has described a number of observations of grenz ray:
I.
Thickness of epidermis particularly the stratum corneum affects the reaction:
A dose producing no visible reaction in a thick well-pigmented epidermis may result in marked erythema in a thin-skinned person.
Body areas arranged in order of decreasing sensitivity are
1.
Eyelids
2.
Neck, popliteal and antecubital fossae, female breasts
3.
Flexor thighs, arms, chest and abdomen
4.
Dorsal fingers, hands, toes, feet
5.
Face (unless pigmented)
6.
Back, extensor extremities
7.
Nape of neck
8.
Palms
9.
Soles
10.
Scalp (sensitivity depends on the amount of hair)
5.3.5 High-Dose (>10 Gy) Effects
Kalz [18] describes a triphasic erythema response with doses greater than 10 Gy hvl 0.02 mm Al:
1.
Early erythema appears within a few hours, increases for 24 h and fades quickly.
2.
Second wave reaching peak within 10–14 days and persists for 3–4 days.
3.
A third and more intense erythema (main erythema) occurs between 24th and 34th day lasting 5–7 days – occasionally the erythema waves may coalesce or second wave may not appear at all. If a main erythema develops, then erythema may recur with heat suggesting vascular damage. If the dose is fractionated, then main erythema can be avoided and clinical experience indicates that late sequelae will not appear.
5.3.6 Effect on Pigmentation
Pigmentation: the relationship between dosage and pigmentation is less definite – but usually disappears spontaneously within 4–12 weeks. Lentigo like spotty pigmentation can be seen with overdosage.
5.3.7 Nail Transmission
Gammeltoft and Wolf who have examined transmission of 12 kV grenz rays through normal and diseased nails found that normal nails transmitted about 30 % [11].
5.3.8 Effect on Psoriasis
The mechanism of benefit for psoriasis is unknown but it is speculated that it may be similar to the anti-inflammatory mechanisms demonstrated for low-dose radiation (<1 Gy): modulation of cytokine and adhesion molecule expression on activated endothelial cells and leukocytes and of nitric oxide production and oxidative burst in activated macrophages and granulocytes [40].
5.3.9 Cancer Production
Cutaneous neoplasms in rats have been produced with grenz rays: effective single doses ranged from 50 to 90 Gy; effective weekly (3–6 Gy) schedules totalled 78–264 Gy. The amount of grenz ray was greater (possibly five times) than that required by 80 kV X-ray [55]. In mice, squamous cell carcinoma was induced by grenz ray 0.5 G daily 5 days per week to total of 300 Gy [44].
5.3.10 Effect on Melanocytes
Nakatani and Beitner [34] studied melanocytes after irradiating with 4 G grenz ray weekly for 3 weeks compared with UV-A 30 j/cm2: ultrastructural changes were an increase in the number of premature and mature melanosomes, elongation and protrusion of cytoplasm and sometimes indented nuclei – the qualitative changes were similar to UVA.
5.3.11 Overdose
5.4 Equipment
1.
Progressus Medica AB makes new grenz ray machines. The tube has a beryllium window 0.65 mm thick. Although the tube is rated for 50 kV, it operates at 9.95 kV. The unit has six cones 1–12 cm diameter, operates at a focal skin distance 17 cm, has a computer controlled timer and produces audible signal when X-rays are produced. www.progressusmedica.se
2.
Xstrahl make Xstrahl 100 unit which can provide superficial X-ray and grenz ray therapy (formerly Gulmay D3100). The unit can be configured to provide a number of X-ray qualities. The author uses this machine configured to deliver HVL 0.033, 0.047, 0.7, 1 and 2 mm Al. It comes with a set of standard cones (now advertised to give range of 1–15 cm field size diameter), but we have a custom cone 18.7 cm diameter (to simulate the 20 cm diameter square cone available with the Philips RT100 machine which allows treatment of whole palm and sole) (Fig. 5.3).
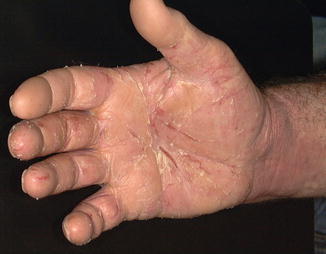

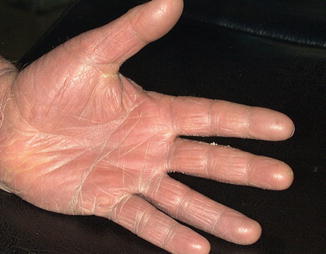
Fig. 5.3
Right hand dermatitis pretreatment
3.
Old units: Philips RT100 (capable of delivering HVL 0.047 mm Al) and other old units may be able to be obtained from oncology centres or by word of mouth from members of the International Dermatologic Radiotherapy Society.
5.5 Safety Requirements
Grenz rays as a form of ionising radiation may have acute effects on skin generally mild with normal treatments (erythema, burning sensations, tanning and blistering) and more severe in overdosage, (atrophy, telangiectasia, crusting, erosions) [22] and potential long-term effects of carcinogenesis (to be discussed later). However, if guidelines are followed, then grenz ray can be given safely. Warner and Cruz [52] have proposed the following recommendations for safe and effective administration of grenz ray (based on their review of the literature) which I will add to:
1.
There should be an established diagnosis.
2.
Grenz rays should only be used in refractory cases when treatment failure consequences are unacceptable or alternatives not accepted by or tolerated by patients.
3.
Grenz rays should only be used when there is a reasonable expectation that treatment will be helpful (inflammatory conditions where pathology is within the absorption range or previous literature reports of effectiveness).
4.
Grenz rays should not be used in children (I would add not in pregnant patients – primarily for medicolegal reasons).
5.
Grenz rays should only be given by trained personnel.
6.
Meticulous radiation protection should be used: operators should stand no closer than 4 m when grenz rays are delivered – ideally the machine should be in a proper shielded treatment room with operating controls outside the room and with interlock doors – this requirement will probably be mandated by governing bodies. Cones should be used (if they cannot, then protective measures as for superficial radiotherapy should be used. The use of cutouts may produce well-defined field edges which may exaggerate the appearance of pigmentary changes).
7.
Patients should be questioned re previous radiation exposure and exposure to other potential carcinogens.
8.
No topical agents should be applied to the treatment areas on the treatment day prior to irradiation to avoid irritation or reduced efficacy.
9.
Radiation dose should be adjusted for the treatment site’s sensitivity to grenz ray. Palms, soles and scalp can tolerate 2–4 Gy per treatment, other sites generally 2 Gy and anogenital area 0.5–2 Gy. Adjustment of dosage due to the presence of hair which absorbs grenz ray has been recommended by Wulf et al. [54] by multiplying dose by 1.5–3 times based on the assessment of thin or thick hair layer – this advice I believe should be taken cautiously – I would not give more than 4 Gy per treatment.
10.
While the US literature recommends 50 Gy lifetime cumulative dose per treatment area, Lindelöf [25] (one of the authors of the only large-scale study of carcinogenic effects of grenz ray [27]) believes higher doses can be tolerated: he recommends 100 Gy maximum cumulative dose; although if higher doses are required, patients should be monitored closely, dose should be fractionated four to six treatments once per week with 6 months rest between courses and the palms, soles and scalp can safely tolerate more than 100 Gy per lifetime. I believe that areas that are not routinely exposed to other carcinogens (especially UV light) such as the palms and soles are at less risk of subsequent cancer and that although lifetime doses should be kept under 100 Gy, higher doses on the palms and soles can be considered provided that Lindelhof’s suggestions are followed. Although scalp tolerates grenz ray well, I hesitate to consider exceeding 100 Gy lifetime dose in view of the potential for this area to be exposed to UV (especially in patients with alopecia).
5.6 Practical Aspects
At the Skin and Cancer Foundation, Victoria, we have two X-ray machines capable of providing ultrasoft X-rays: our original machine is a Philips RT 100 which has a 20 cm square cone suitable for treating soles in one field and a Gulmay D3100 (now renamed as Xstrahl 100) with standard cone sizes and custom cone 18.7 cm diameter. The former machine is calibrated to give hVL 0.047 mm Al, the latter hVL 0.047 and 0.033 mm Al. We use hVL 0.047 mm Al routinely. We use a treatment schedule similar to that of the Karolinska Institute: four to six weekly treatments of 3 or 4 Gy for palms and soles, 1–2 Gy for other areas.
5.7 Clinical Aspects
Grenz ray is indicated for treatment of a variety of inflammatory skin disorders: eczema, psoriasis, palmoplantar pustulosis, neurodermatitis and, pruritus ani, et vulvae. It has also been reported for lichen planus, Grover’s disease, Darier’s disease and histiocytosis X [35]. It was used in the pre-antiviral era for herpes simplex [35] I have used grenz ray for Shamberg’s disease and erythema elevatum diutinum. Grenz ray has been reported to soften skin in generalised morphea [33] and to decrease lesions and itch in pruritic disseminated superficial actinic porokeratosis [39].
Grenz ray treatment of acne vulgaris [41] has been superseded by other therapies and is not recommended.
Grenz ray has been used for treatment of actinic keratoses and Bowen’s disease.
Some of these indications will be discussed in more detail.
5.7.1 Hand Eczema/Dermatitis
There is no generally accepted classification of hand eczema and a paucity of controlled trials of any treatment for this common skin disease [49].
A double-blind study of grenz ray in chronic eczema of the hands [31] showed a significantly better response to active treatment 5 and 10 weeks after commencement of treatment compared with untreated control utilising treatment schedule at the Karolinska Institute.
Lewis reports using 2 Gy dorsum of hands and 3 Gy palms weekly two to three doses for resistant adult atopic chronic hand eczema [23].
Cartwright and Rowell found that treatment of chronic hand eczema with grenz 3 Gy every 3 weeks for a total 9 Gy was no better than placebo (this treatment schedule is not usual) [4].
Fairris compared superficial X-ray to grenz ray therapy: 1 Gy superficial compared with 3 Gy grenz given three times at 3-week intervals and found that both produced clinical improvement although superficial X-rays were more efficacious [6].
Schalock et al. reported their patient’s perception of treatment of recalcitrant dermatoses with grenz ray – 29 % had dermatitis – 65 % of these had treatment of the hands with 66 % reporting decreased severity or resolution [42].
Walling et al. has reported complete remission and no recurrence for 48 months of frictional hyperkeratotic hand dermatitis in a dermatologic surgeon [51].
A quality assurance analysis of ultrasoft X-ray (hvl 0.047 mm Al) treatment at the Skin and Cancer Foundation, Victoria [53], for treatments given from 2003 to 2009 was conducted, and patient’s perception of treatment was recorded by standardised telephone questionnaire. One-hundred fifty patient responses were obtained (total number of treated patients was 259) for a total of 628 fields treated. Dermatitis was the diagnosis in 42.3 % patients who responded.
Two-hundred forty-five dermatitis fields were treated with 137 clearing, and 71 much improved (206/245 fields were hands). One-hundred thirty-eight fields could be evaluated for duration of response: 10 had never recurred, 69 within 6 months, 17 6–12 months and 42 after 12 months.
Hanfling and Distelheim performed a comparative study comparing grenz ray with superficial X-ray in 24 patients with various forms of dermatitis and showed 21/28 had similar response and 6/7 had better response to grenz ray [12].
King and Chalmers showed statistically significant improvement in chronic hand dermatitis with superficial X-ray at 1 month after treatment [20], but this difference was not present at 6 months, and Duff et al. showed benefit with megavoltage therapy for chronic vesicular dermatitis with 47 % complete resolution, 53 % decreased severity [5].
Sheehan-Dare et al. compared topical photochemotherapy (PUVA) given three times per week for 6 weeks to superficial radiotherapy 0.9 Gy 50 kV 1 mm Al added filter given three times at 3 week intervals [45]. The mean clinical severity scores showed significant improvement over pretreatment scores for both treatments – radiotherapy significantly better than topical PUVA at 6 weeks but not at 9 and 18 week assessments. The symptom severity scores were lower for superficial X-ray treated compared to topical PUVA at 9 and 18 weeks.
Sumilia et al. reported 22 patients with therapy-resistant eczema and six with psoriasis treated with 43 kV or 50 kV radiation for a total of 88 fields which showed reduction [45] or complete remission [40] in symptoms in 83/88 fields treated with 62/88 maintaining benefit at last follow-up (median 20 months – range 4–76 months) – 32 with complete remission [48]. There was no difference between single doses of 0.5 Gy (median total dose of 5 Gy) and 1 Gy (median total dose of 12 Gy).
In summary, although it is difficult to compare these different studies, there is evidence that:
1.
Grenz ray given in treatment schedules as performed at the Karolinska Institute is helpful for refractory hand dermatitis.
2.
Superficial X-ray radiation and megavoltage radiation are also helpful and may be more effective than grenz ray because of greater penetration. Grenz ray has the advantage of greater safety (less risk of carcinogenesis and late radiation changes) and can be repeated.
3.
Get Clinical Tree app for offline access

Ultrasoft X-ray is at least as helpful as grenz ray given in treatment schedules as performed at the Karolinska Institute and may be more so (although this remains to be proven) (Figs. 5.4, 5.5, 5.6, 5.7, 5.8, 5.9 and 5.10).
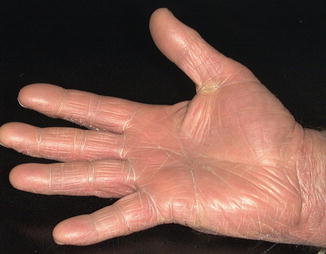
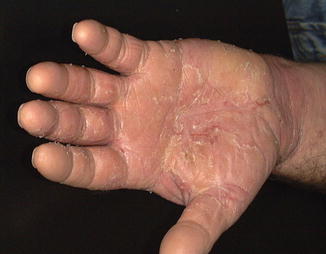

Fig. 5.4
Right-hand dermatitis posttreatment

Fig. 5.5
Left-hand dermatitis pretreatment