Fig. 22.1
(a) Posterior band slippage with gastric prolapse. (b) Anterior band slippage with gastric prolapse. (c) Concentric pouch dilatation
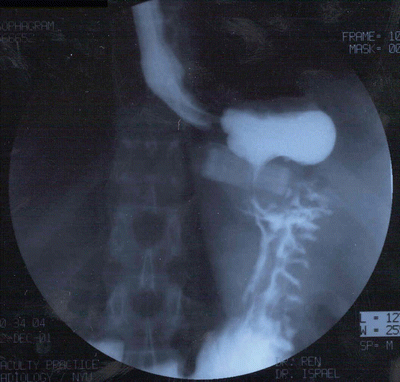
Fig. 22.2
Esophagram film of anterior band slippage with gastric prolapse
Treatment of gastric prolapse is operative, although complete deflation of the band may temporarily improve symptoms. The band may be simply repositioned laparoscopically if the slip is acute and the herniated fundus is able to be reduced and replicated. Some of the devices, such as the Lap Band APS System™, may be opened. This feature allows more easy reduction of the prolapsed stomach. Another option is to remove the band, take down the previous plication, form a new retro-gastric tunnel, and place a new band. If there is a significant amount of edema of the pouch, a larger sized band may be chosen, if available. In some instances, there may be so much dilation or edema of the pouch above the herniated fundus that band replacement is not possible. In this case, the band should be removed, and after a period of 6 weeks, the band can be replaced after the swelling has subsided.
In the face of a gastric prolapse, conversion to another bariatric surgical option, such as sleeve gastrectomy or Roux-en-Y gastric bypass, is possible; Ponce et al. have shown that by salvaging the band, a patient will preserve most of their initial weight loss and comorbidity resolution [9]. Finally, it is important in every reoperation of a band patient to treat any hiatal hernia that may be unmasked.
One worrisome feature of the gastric prolapse is the rare possibility of ischemia or necrosis of the herniated fundus. Generally, the slip does not cause severe pain. A patient complaining of severe abdominal pain, especially when out of proportion to the physical exam, may be developing ischemia of the fundus and warrants prompt operative exploration. If full-thickness necrosis is identified, the band should be removed and the necrotic stomach resected. If ischemia without necrosis is seen, the fundus should be reduced and observed in the operating room to assess for viability (Fig. 22.3).
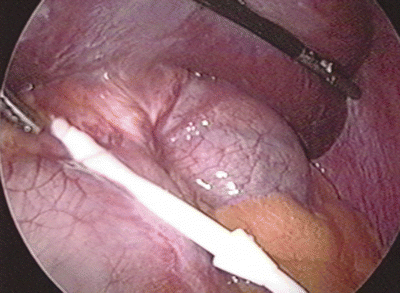

Fig. 22.3
Ischemic fundus secondary to acute gastric prolapse
Band Erosion
Band erosion has been reported to occur between 0.2 and 32.6 % [10] and appears to be associated with surgical technique, either from sewing the stomach over the band buckle or from micro-perforation or deserosalization of the stomach during surgery. The European experience of a 15–32 % erosion rate appears to be related to both surgical technique of using the perigastric technique in the early experience and low surgical volumes [5]. Surgeon experience directly correlates to erosion rate [9]. Typical presentation is delayed port infection, lack of restriction with resultant increase in appetite and decreased satiety despite maximum band adjustment, abdominal pain, or loss of fluid from the device system. Patients generally are not systemically ill due to the confinement of the gastric perforation. Although an esophagram may demonstrate suspicion for a band erosion with barium traversing around the band (Fig. 22.4), endoscopy confirms band erosion and necessitates band explantation (Fig. 22.5). The approach to band removal is determined by the extent of the band eroded into the stomach lumen. The current recommended approach is laparoscopic band removal and repair of the gastric wall with drainage. One approach is to mobilize the band completely, divide the buckle, remove the band, and repair the gastric wall with interrupted permanent sutures, with or without omental reinforcement of the repair. An alternative approach is to create a separate anterior gastrotomy to remove the band from the inside of the stomach with subsequent primary continuous repair of the gastric wall. This technique allows undisturbed healing of the band site without sutures through the inflamed friable gastric wall. However, this requires a minimum of 50 % of the band to be eroded, including the buckle; otherwise, it is technically difficult to remove the band. Similarly, endoscopic removal of the band has been described, but requires the buckle of the band to be eroded and special endoscopic instruments that are presently not available in the USA. In addition, a separate operation is required to remove the port and tubing [11]. Open laparotomy should be used when the prior approaches cannot be performed, or if on rare occasion, the patient has internal bleeding. Reports of band erosion through the left gastric artery have been published and should be removed by laparotomy in order to ensure vascular control [12]. After band removal, weight gain is typical and most patients will require another operation. Another band can be placed without increased risk of repeat band erosion, but should not be performed simultaneously with removal of eroded band [13]. A minimum of 3 months should pass to allow for inflammatory reaction to resolve. The risk of erosion appears to be minimized by using the pars flaccida technique, plicating the stomach loosely over the band, not covering the buckle with the stomach and treating all port infections early.
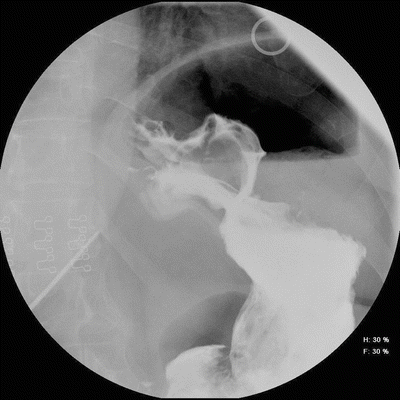
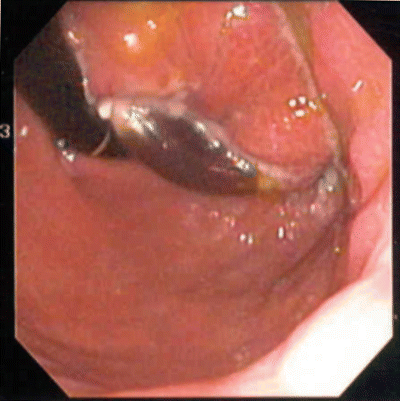

Fig. 22.4
Esophagram film of band erosion. Barium flows through and around gastric band

Fig. 22.5
Endoscopic view of eroded gastric band, which is discolored
Device Malfunctions
Device malfunction secondary to a leak is not common and not lethal, but is detrimental to weight loss due to failure of the band adjustment mechanism. The incidence is <1 % [3]. These problems include tubing break (Fig. 22.6), tubing disconnection, port puncture, and band puncture. The tubing should pass in a smooth line from the port located on the anterior abdominal wall, into the abdominal cavity. Acute angles in the tubing can lead to kinking and device fatigue of the tubing (Fig. 22.7). Thick muscular abdominal walls or suturing of the fascia tightly over the tubing can cause “wearing away” of the tubing wall (Fig. 22.8). Intra-abdominal scar tissue causing the tubing to bend acutely can also contribute to material fatigue. Care should be taken during the surgery not to inadvertently puncture the band with the needle nor to handle the device with traumatic instruments. Likewise, care should be taken during band adjustments not to inadvertently puncture the port neck; this is the most common cause of device malfunction.
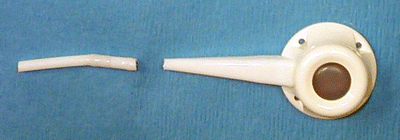
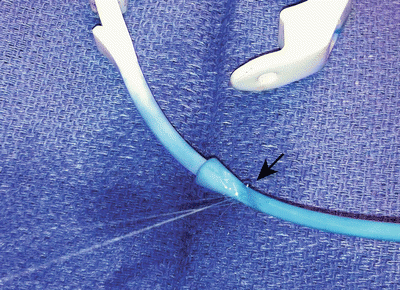


Fig. 22.6
Tubing break

Fig. 22.7
Leak from tubing secondary to external compression

Fig. 22.8
Leak secondary to wearing down of silicone overlying metal connector
Locating the area of leak radiographically is typically unsuccessful, may be misleading, and is generally not considered to be necessary. An abdominal X-ray can show tubing disconnection from the port (Fig. 22.9). Contrast dye injection into the port with subsequent fluoroscopy or X-ray is not reliable due to the common tendency for the contrast to drip alongside the tubing before it extravasates. In addition, the rate and volume of contrast extravasation are usually too small to capture in an obese individual. If tubing disconnection is ruled out, the best way to locate the leak is by surgical exploration. Injection of 50 % dilute methylene blue into the port in the preadmission area will allow enough time for tissue staining surrounding the leak (Fig. 22.7




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






