Study
Recurrence rate
95 % CI
% weight
Lower
Upper
Excision
Pitman [34]
0.09
0.01
0.29
2.31
Hill [35]
0.02
0
0.08
3.05
Argawal-Antal [36]
0
0
0.04
3.2
Osborne and Hutchinson [11]
0.2
0.12
0.3
3.14
Malhotra et al. [25]
0.03
0.01
0.07
3.34
Bub et al. [30]
0.05
0.01
0.13
3.02
Huilgol [37]
0.02
0.01
0.06
3.37
Mahoney [38]
0
0
0.28
1.72
Jejurikar [39]
0
0
0.08
2.79
Walling et al. [29]
0.07
0.02
0.2
2.78
Lee [40]
0.06
0
0.3
2.04
Abdelmalek [41]
0.02
0
0.04
3.45
Chin-Lenn [42]
0.08
0.03
0.15
3.19
Akhtar [43]
0.03
0
0.1
3.06
Hou et al. [26]
0.06
0.03
0.09
3.47
Joyce et al. [6]
0.02
0.01
0.04
3.53
Matos [44]
0.41
0.34
0.48
3.43
Rawlani [45]
0.22
0.06
0.48
2.14
Random pooled rate (Excision)
0.05
0.02
0.1
53.05
Mohs
Robinson [27]
0.06
0
0.3
2.04
Cohen [46]
0.03
0
0.14
2.73
Clayton [47]
0.01
0
0.07
3.12
Bienert [48]
0
0
0.05
3.11
Bricca et al. [7]
0
0
0.02
3.50
Bhardwaj [49]
0
0
0.03
3.42
Temple [50]
0.03
0.01
0.06
3.42
Walling et al. [29]
0.38
0.15
0.65
2.04
Bene [51]
0.01
0
0.05
3.34
Kunishge [52]
0
0
0.01
3.60
Chin-Lenn [42]
0.05
0.01
0.14
3.00
Newman [53]
0.02
0
0.04
3.47
de Vries [54]
0.04
0.01
0.10
3.23
Etzkorn et al. [4]
0
0
0.01
3.56
Hou et al. [26]
0.03
0.01
0.07
3.36
Random pooled recurrence rate (Mohs)
0.01
0
0.03
46.95
Overall random pooled recurrence rate
0.04
0.02
0.06
100
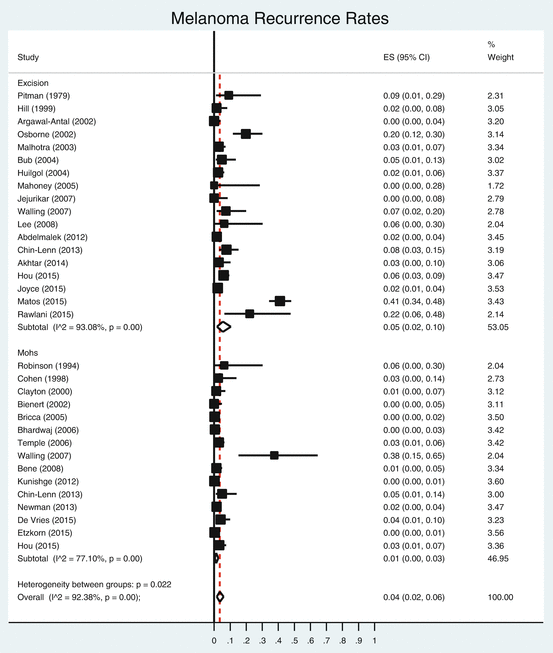
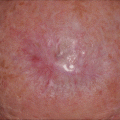
Fig. 15.1
Forest plot of the recurrence rates for melanoma, stratified by procedure type (excision vs. Mohs)
Defining Local Recurrence
Local recurrence of LM/LMM typically presents as hyperpigmented macules or patches along a scar or within a graft in the area of prior intervention as depicted in Fig. 15.2.
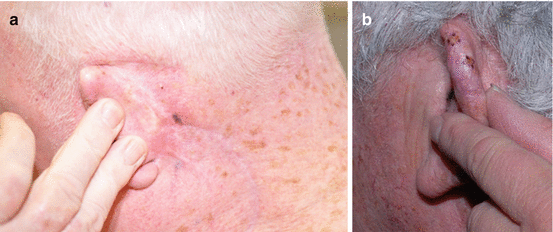

Fig. 15.2
(a) Recurrent LMM at the margin of the previous surgical scar. (b) Recurrent LM in a graft (hyperpigmented macules marked in purple)
The exact definition of LR for LM/LMM varies from publication to publication. Most commonly, LR for LM/LMM is defined as hyperpigmentation just along a prior surgical scar from primary treatment to LM within 5 cm of the initial scar [4, 5] with additional definitions in the medical literature. LR can present a challenge to define in a cancer that by definition tends to occur in severely sun-damaged skin; field damage with subsequent primary lesions occurring in close proximity to the initial lesion is not uncommon. Figure 15.3 illustrates this challenge.
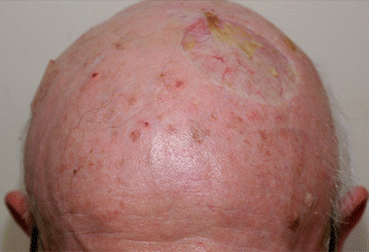

Fig. 15.3
Patient with extensive photodamage who developed multiple primary melanomas on the scalp
The authors prefer the definition of a subsequent biopsy-proven melanoma within or adjacent to the surgical scar, within the site of the initial surgical defect, consistent with studies where a definition was reported [4, 6–8]. Clinical photography can be essential in determining whether a lesion should be classified as a local recurrence. Standard follow up examination of patients with a history of LM/LMM must include both inspection of the prior treatment site/scar as well as a thorough exam of the adjacent and/or draining lymph node basin. While the interval for follow up varies depending on suspected risk of recurrence (e.g. shorter follow up intervals for patients with more invasive disease), at least annual follow up is recommended for any patient with a personal history of melanoma [9].
Monitoring for Local Recurrence
Monitoring for recurrence of LM poses several special challenges. First, lentigo maligna occurs on skin with chronic actinic field damage. Therefore, patients are also predisposed to development of additional primary melanomas in the initial treatment area due to the field effect of chronic cumulative ultraviolet radiation exposure in that specific region, as seen in Fig. 15.4.
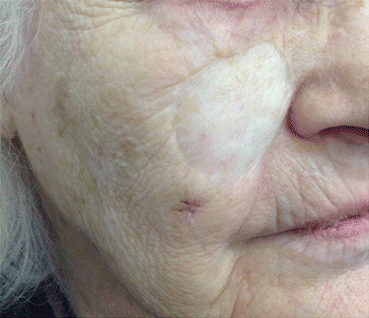

Fig. 15.4
This 88 year old woman developed a second primary lentigo maligna melanoma just inferior to the graft scar of a lentigo maligna treated 7 years prior.Her background skin demonstrates extensive photodamage
While benign lentiginous hyperpigmentation may occur within a skin graft (Fig. 15.5), or along the scar of a prior LM [10], the clinician should maintain a high index of suspicion for LR, as recurrence rates can be as high as 20 % in those treated with standard wide local excision [11], and even higher with other techniques such as laser and cryotherapy [12]. As hyperpigmentation cannot be readily differentiated from recurrence (Figs. 15.5 and 15.6), a histopathologic diagnosis is recommended in this situation.
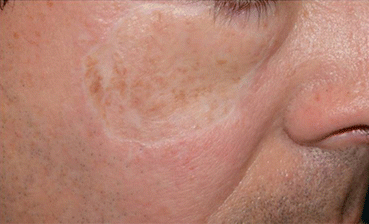
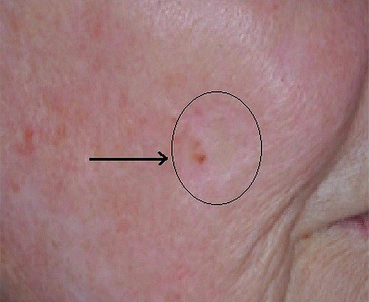

Fig. 15.5
Benign lentiginous hyperpigmentation in a skin graft following reconstruction

Fig. 15.6
Recurrent LM within a graft, eight years following the initial surgery
Few studies have demonstrated long-term recurrence rates in patients treated with topical therapies (i.e. imiquimod); however, failure of treatment has been reported in as many as 23 % of cases when long-term follow up is provided [13]. Recurrence of previously pigmented LM as amelanotic LM presenting as erythematous dermatitic plaques has also been described, necessitating a high index of suspicion for any new lesion or suspected dermatitis in a prior surgical site for LM [14, 15].
Recurrence of Lentigo Maligna as Invasive or Metastatic Disease
The most pressing concern when monitoring for recurrence of LM/LMM is presence of invasive melanoma or lymph node metastasis as the first sign of recurrence. Case reports exist of LM, previously thought to be in situ, treated nonsurgically and recurring as invasive or metastatic disease [16, 17]. For example, Woodmansee and colleagues reported a case of a biopsy-proven LM treated nonsurgically with imiquimod with clinical and histologic resolution of tumor from sampling biopsies. Approximately 2 years following treatment, the patient developed nodules in the previously treated area, which were biopsy-proven to be 1.13 mm depth melanoma. As is the case with typical nonsurgical treatment, a tumor debulking specimen was not initially sent for histopathologic examination, suggesting that there may have been an undetected microinvasive component, later developing into clinically evident invasive disease. This patient went on to develop both pigmented and nonpigmented subsequent recurrences [16]. Fisher and colleagues reported a large recurrent lentigo maligna that underwent 13 pretreatment biopsies, all confirming in situ disease, and treated with topical imiquimod and showing initial clinical response. However, multiple invasive satellite nodules up to 3.3 mm in Breslow depth developed at the periphery of this lesion, and on Mohs surgery, a tumor debulking specimen showed only melanoma in situ in the central lesion. Fortunately, the patient had surgical removal of the invasive disease and remained free of disease at 17 months [17]. Similarly, Guitera and colleagues reported a series of nonsurgically-treated LM with two invasive treatment failures at several months post treatment, with Breslow depths of 0.4 and 0.6 mm, respectively [18]. For this reason, the authors recommended surgical treatment if possible for any recurrent lesion, to rule out occult invasive disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree