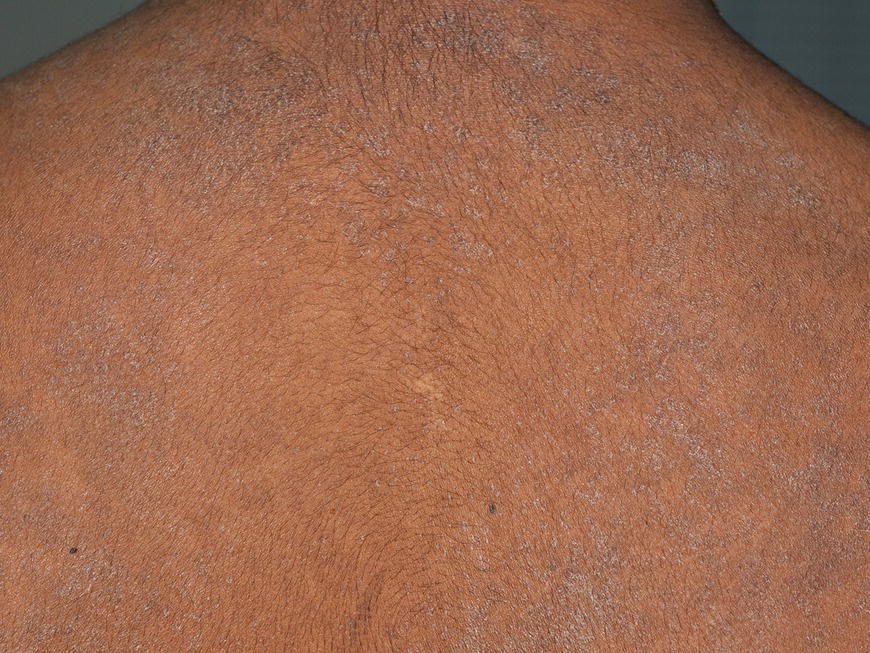
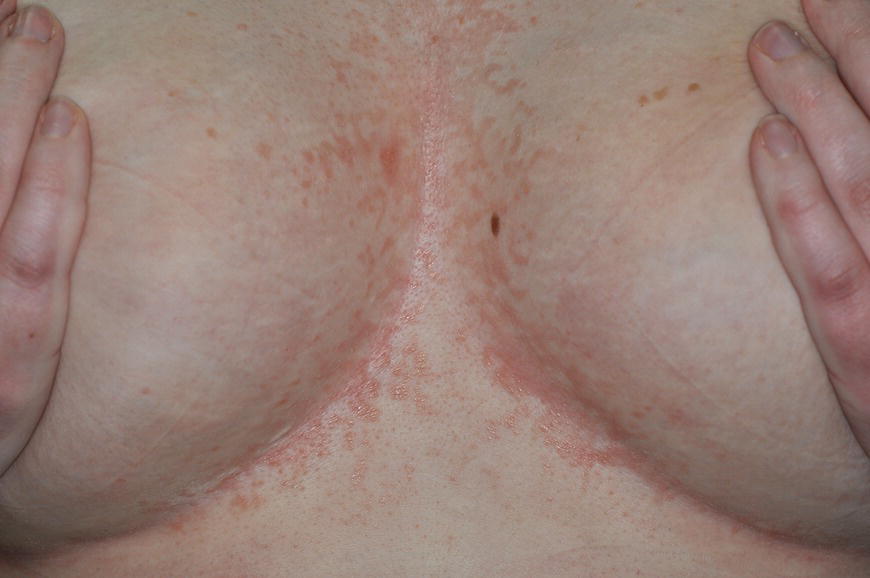
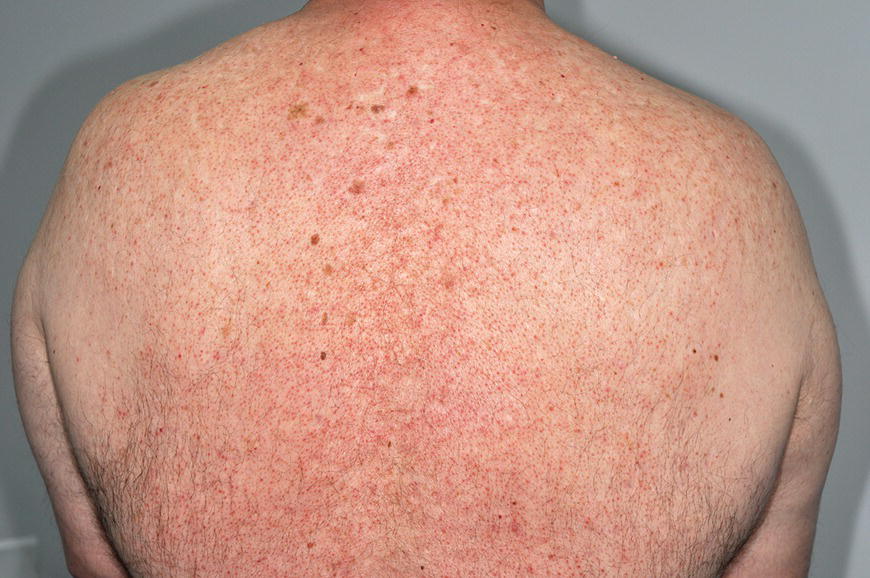
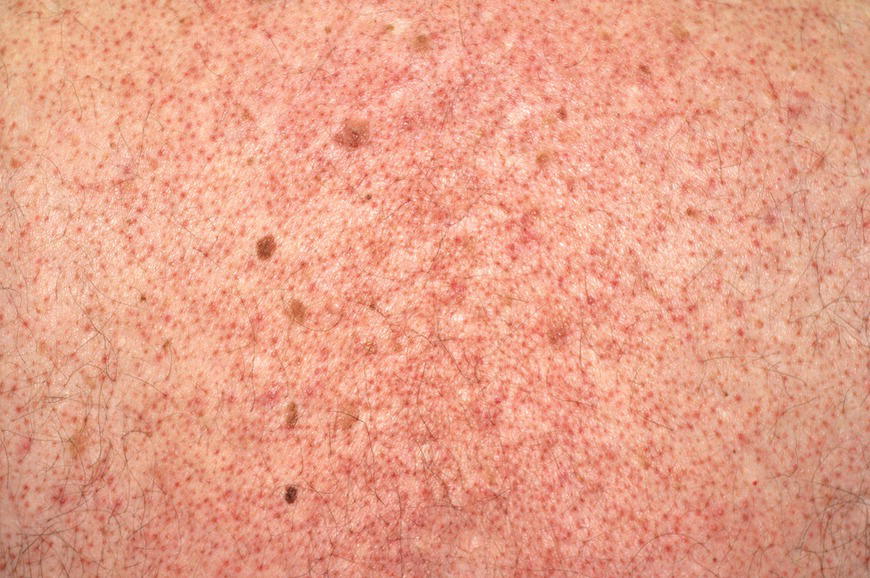
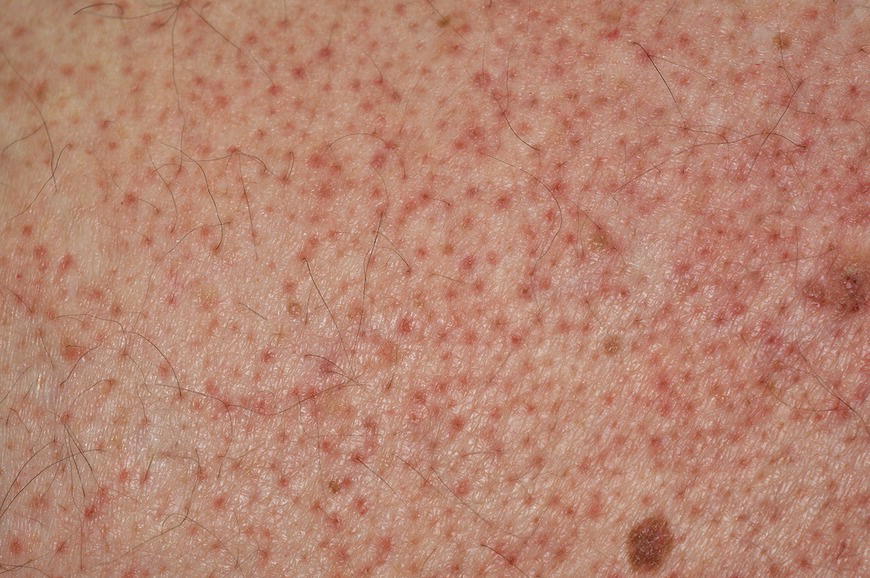
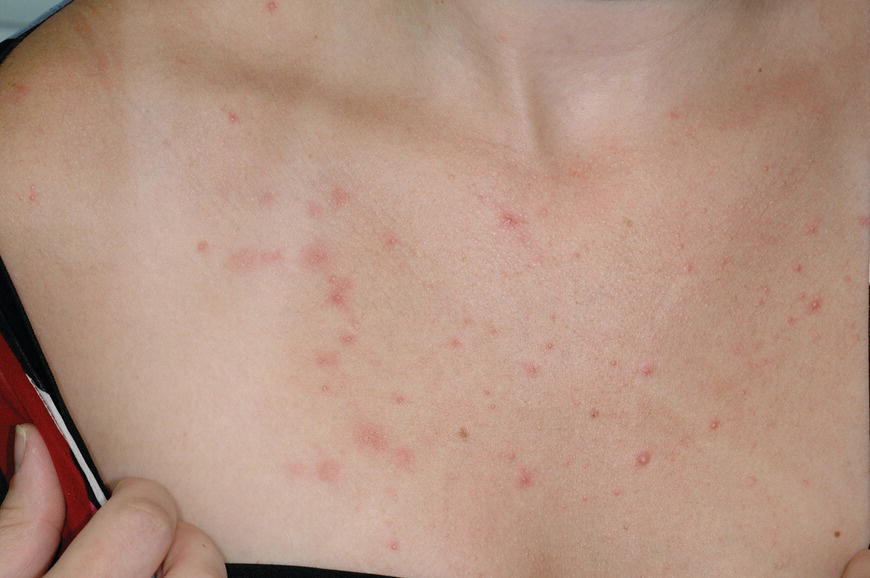
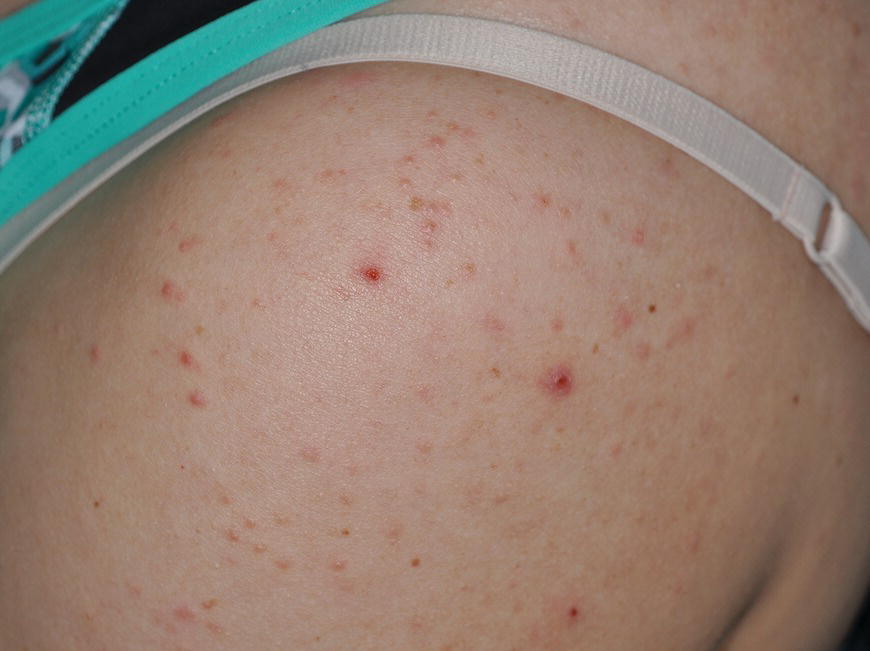
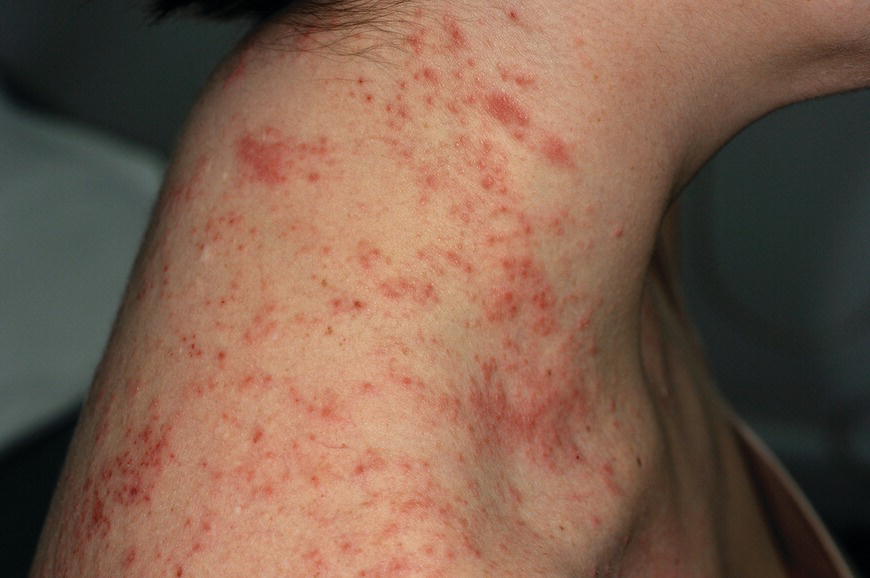
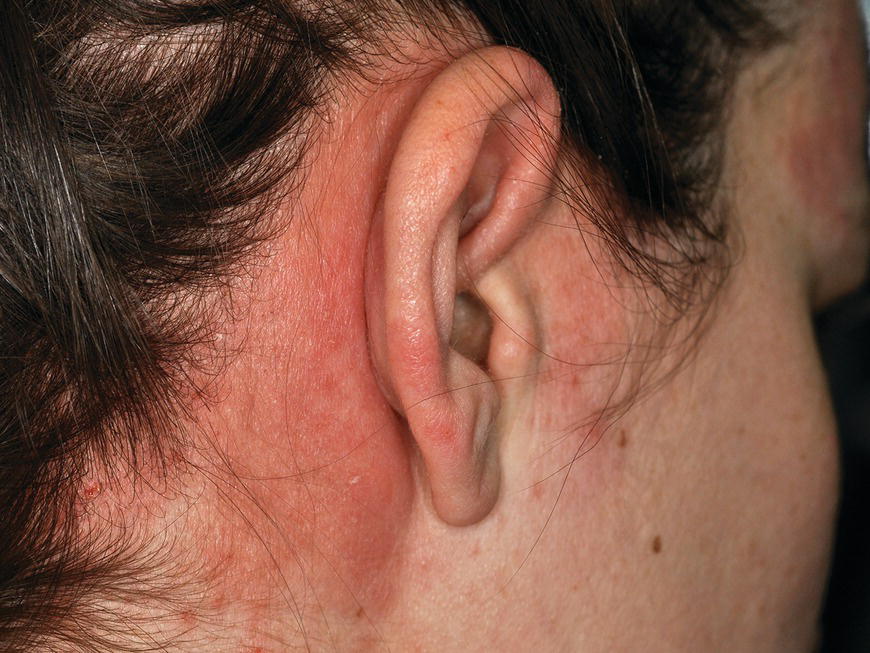
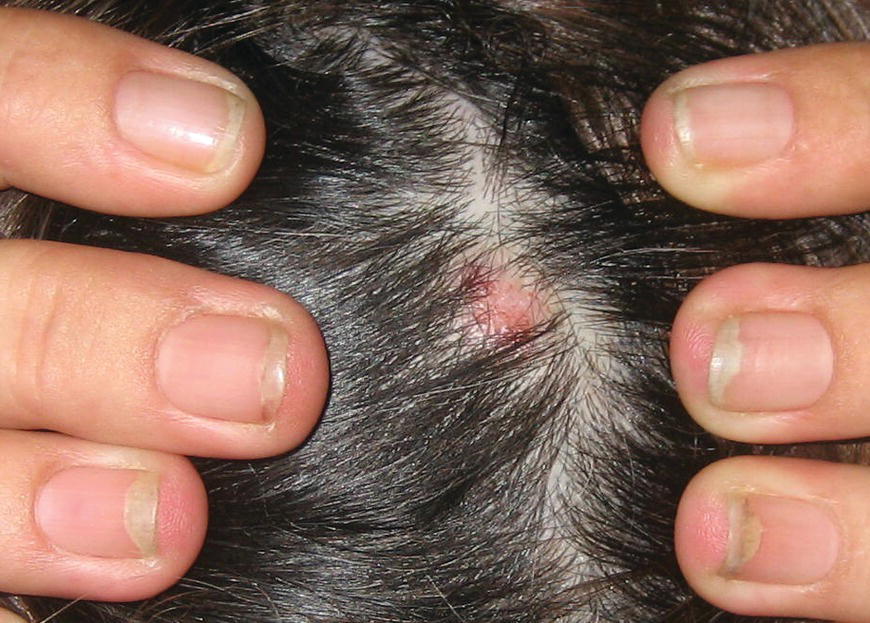
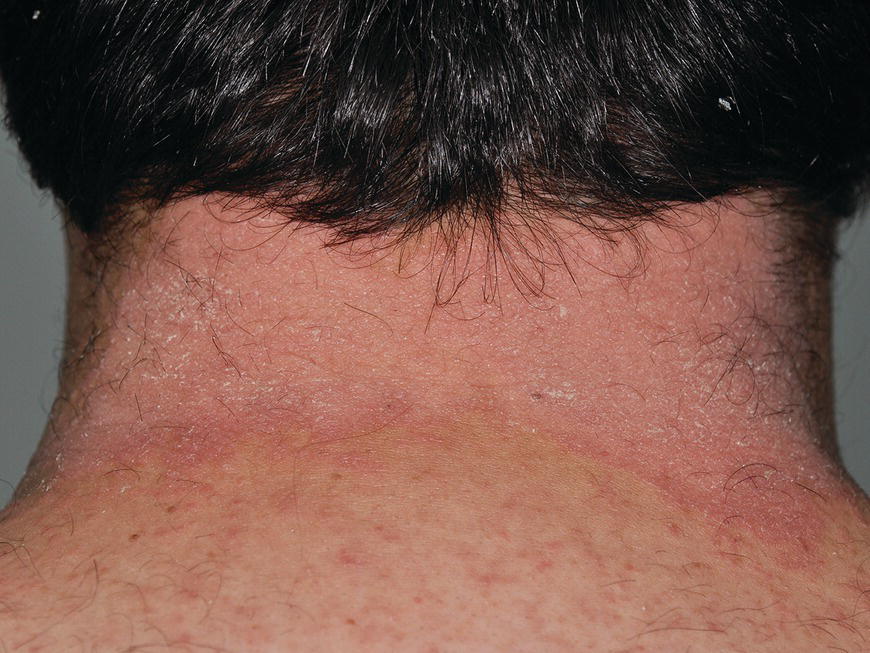
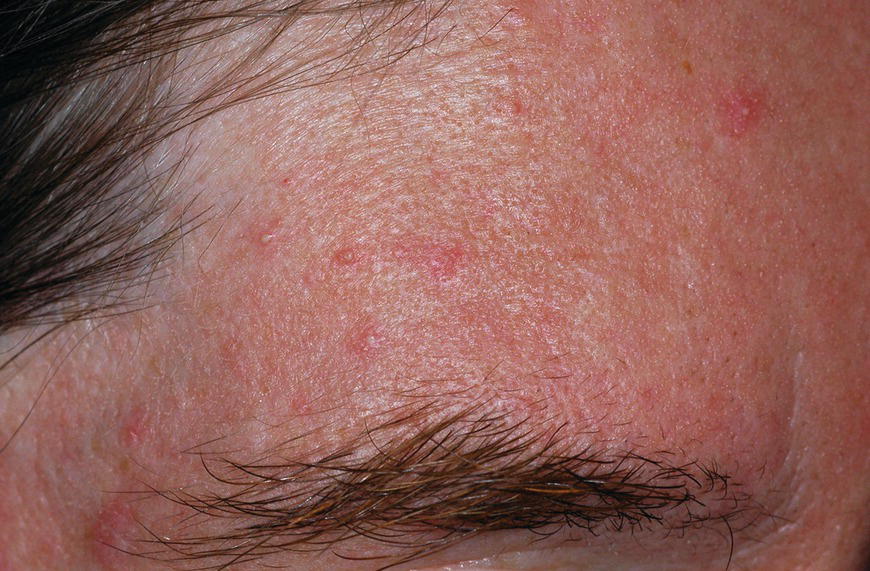
Chapter 6 The inflammatory reaction that causes the trouble in all the acnes is directed at a limited number of foreign materials. Remember that foreign is defined as anything that is not supposed to be in the dermis, and the dermis is that part of the skin that is below (on the dermal side of) the basement membrane. And remember that the basement membrane runs horizontally under the epidermis but dives deep and wraps around the epidermal appendages, all of them, from sweat glands to hair bulbs. It is thin in some areas, thicker in others. It follows the contours of the folliculopilosebaceous unit (FPSU) like a vinyl glove on your fingers. It provides support to the appendages. It anchors the epidermis to the dermis. It is a semipermeable barrier, allowing limited amounts of water, chemicals, and a few mobile cells to cross into and out of the epidermis and the appendages. (See Figure 2.7.) A recently described chemical messenger system, hypoxia-inducible factor 1 (HIF-1), may be responsible for the two processes that are active at this point in the process of acne development. HIF-1 appears to be able to induce “hyperproliferation and incomplete differentiation of epidermal keratinocytes” [1]. It is also “a major regulator of cellular adaptation to low oxygen stress” and “plays an important role in cytokine production by keratinocytes and in neutrophil recruitment to the skin” [2]. Thus, it may stimulate the overgrowth to bursting and recruit inflammatory cells to migrate to the area in response to the anoxic stress. Once the barrier is broken, foreign material that is located in the ducts of the FPSU becomes “visible” to the body’s immune systems. This can happen if the immune cells find their way through a split in the basement membrane into the follicular duct, or if the materials inside the duct find their way out through a leak. Either way, the immune systems recognize the foreign material, and this is the first trigger to inflammation. If and when the immune reactions proceed, the duct leaks even more and often ruptures, and greater volumes of materials in the duct find their way out into the dermis. There they trigger the numerous inflammatory processes of the innate and adaptive immune systems, and so the battle intensifies. To cool and clear acne, we must know all the materials stuck down in the duct that are causing the inflammation. Then we can plan to eliminate each and every one of them. Bacillus acnes, the “acne bacillus,” was first described by Gilchrist in 1900. It was renamed Corynebacterium acnes in 1909 and later Propionibacterium acnes (P. acnes). There are 22 members of the Propionibacterium family, but P. acnes (which comprises several strains) appears to be the only important one in acne. As such, it has been the target for elimination by dermatologists for decades. But there is a problem. Simply overwhelming the population of P. acnes with antibiotics doesn’t usually clear acne. This simple fact should have given us a hint, decades ago, that something else was going on. More on that below. While it has been generally accepted that P. acnes is a normal organism on everybody’s skin (a commensal), that is not the whole story. Recently, with a simple but sophisticated technique, Bek-Thomsen showed that P. acnes seems to have exclusive rights of occupancy to the FPSU. His work shows that no other organism can make that claim [3]. Furthermore, it was suggested that this relatively harmless organism actually has a role as a gentle guardian of the integrity of the FPSU, a concept that has found support elsewhere [4]. So how does such a protective role really work? Imagine that P. acnes is sitting quietly in a follicle. It is a facultative anaerobe. That means it can survive and multiply in a very low-oxygen (or no-oxygen) environment. Normally, the follicle is well oxygenated so P. acnes’ motor is simply “idling in neutral.” If there is a minor injury to the duct, like a scratch or a rub, a little bit of P. acnes antigen may leak out of the duct. Or, much less likely, perhaps a wandering dendritic cell bearing toll-like receptor 2 (TLR2) may gain access to the ductal lumen. If such contact is made between P. acnes and the innate immune system, the inflammatory cascade gets to work, and normally this initiates activities that repair the damage. One must remember that such low-grade “inflammation” is really designed to return the physical structure of the duct back to normal. The inflammatory system we work so hard to suppress is not all “destruction”—its reparative function is usually ignored, and many of the medications we use will actually compromise this function. Topical steroids, for instance, cause thinning of the skin in hand eczema, a thinning that takes months of steroid avoidance to repair. Barring serious abnormalities (like the overstuffed duct with weak walls in acne inversa), the repair is quickly completed and everything goes back to normal. P. acnes’ role as an immune sentry has been fulfilled. Only if things go terribly wrong is there a hot, destructive inflammatory reaction resulting in permanent damage. We are much more familiar with P. acnes’ role as a pathogen, a bad actor that needs to be eliminated in order to cure the disease. Over the past 60 years, we have brought to bear tetracycline, erythromycin, doxycycline, minocycline, lymecycline, azithromycin, sulfa drugs with and without trimethoprim, clindamycin, clarithromycin, ampicillin, amoxicillin, ciprofloxacin, and even rifampicin. Despite this aggressive attack, we still see the term antibiotic-resistant acne, and that term usually addresses only acne vulgaris. If you add acne rosacea, then metronidazole, neomycin, fusidic acid, mupirocin, azelaic acid, and retepamulin are on the list. Take one step further to acne inversa/hidradenitis suppurativa (AI/HS), and we see that escalation to the “nuclear option” includes long-term systemic rifampicin, moxifloxicin, and metronidazole [5]. It is hard to believe that any bacterial infection could survive that onslaught, and yet only 16 of 28 patients with HS/AI achieved complete remission with up to 12 months of this aggressive triple-antibiotic therapy. We have not yet learned what will occur when the medications are stopped in those temporarily fortunate 16 successfully treated patients. But we can guess. What happens to change P. acnes from a mild-mannered commensal to a “pathogen” able to destroy faces and backs and psyches? And why does our most aggressive antibiotic therapy not work? There are likely four factors at work that bear on P. acnes, and one that has been roundly ignored despite posted warnings. First, P. acnes shifts out of neutral and really gets to work only in an anaerobic (no-oxygen) or a microaerophilic (low-oxygen) environment. So, how does one achieve such an anoxic environment in a healthy teenage face, full of life and the vigor of youth, well vascularized, and supplied with adequate nutrients and all the metabolic systems needed to sustain and repair all normal processes? The answer is possibly, but not proven, that there is simply too much of a good thing available. As described in detail in Section 2.9, increased insulin-like growth factor 1 (IGF-1) and increased insulin and exogenous androgens, added to endogenous steroids and endogenous pubertal IGF-1, overstimulate the follicular ductal keratinocytes. A traffic jam occurs in the follicle: pressure within the confines of the follicular duct compromises the availability of nutrients, especially oxygen. The lack of nutrients diffusing into the area interferes with normal metabolic processes within the keratinocytes. The concurrent anoxia provides a wonderful place for P. acnes to flourish. Nourished anaerobically by the fatty acids of the sebum, P. acnes multiplies mightily, to the point that the colonies are large enough to be easily visible in microscopic sections. When the overstressed follicle leaks or ruptures, the population of P. acnes will have increased by several orders of magnitude, becoming a very potent stimulus of the innate immune system. That is what lights the fire in acne vulgaris. Second, P. acnes has the genomic capacity to support a large number of functions. These enzymatic abilities are not much in evidence when the organism is “idling” quietly in the duct, but under the conditions of anoxia that occur in the compressed confines of the crowded and distended duct, the organism is capable of springing to life at full anaerobic throttle, and the broad panel of metabolic options that the genotype can support apparently become selectively deployed. This shows up as a change in the organism to a fully active reproductive phenotype, triggered by the provision of the anaerobic or microaerophilic environment that the organism prefers. This can be expressed in many destructive ways [6]. These include virulence-associated and fitness traits such as transport systems and metabolic pathways, and the encoding of possible virulence factors such as dermatan–sulphate adhesin, polyunsaturated fatty acid isomerase, iron acquisition protein HtaA, and lipase GehA. The authors argue “that the disease-causing potential of different P. acnes strains is not only determined by the phylotype-specific genome content but also by variable gene expression” [6]. Third, it isn’t really all P. acnes’ fault that such a mess is created. While the genome offers considerable potential for havoc, there is also the reaction to the numerous materials that are explosively released into the dermal and subcutaneous world beyond the basement membrane. These are both the immediate stimulants of the innate immune system and the antigens that the slower acting, yet very potent, adaptive immune response will need to identify, react to, and neutralize. Fourth, the blame for stimulating the immune systems needs serious recognition as a shared responsibility. Not only has the other major intraductal organism been inexplicably ignored, but also the attempts to eradicate this bacterial activity have done wonders to increase the impact of another actor, P. acnes’ silent partner, the yeast Malassezia. Malassezia yeasts were first recognized by Louis-Charles Malassez in 1874, and Sabouraud named the yeast Pityrosporum malassez in 1904. It was not until 1988 that the taxonomists adopted Malassezia as the genus name and combined the ovale and orbiculare forms as Malassezia furfur. There are now 14 species characterized: They are found widely among sebum-secreting animals other than humans. Relationships between specific animal species, specific yeast species, the diseases they induce, and even their geographic human variations are being worked out, but M. globosa seems to be the major contributor to dandruff. Malassezia requires a specific lipid for growth and reproduction, so it is demonstrably lipophilic. Indeed, its need for long-chain fatty acids of carbon chain length greater than 10 (C12–C24) is so profound that positive cultures can be obtained only by adding a source of this material to the culture medium. An olive oil overlay of the Sabouraud culture medium is commonly used. (See Figure 1.8.) While there are several rare Malassezia infections reported in immunocompromised patients, the major widely recognized clinical presentations of Malassezia are as tinea (pityriasis) versicolor (Figures 6.1 and 6.2) and Malassezia folliculitis (Figures 6.3, 6.4, 6.5, 6.6, and 6.7). This yeast’s papulopustular involvement in atopic dermatitis (particularly of the head and neck—see Figures 6.8 and 6.9) [8], in psoriasis (particularly in the scalp—see Figures 6.10 and 6.11) [9], in seborrheic dermatitis [9], and in acne [10] is far from being generally recognized and treated in the general dermatologist’s office or clinic. Figure 6.1 Malassezia growing on the surface is unrecognized by the immune system at first, whether here on the skin surface or down in the pores. Once recognition occurs, this surface infection can become quite itchy and may become pale pink or red. Figure 6.2 The pink inflammation of active Malassezia-induced tinea versicolor. Figure 6.3 Once Malassezia in the pores is recognized by the immune system, an impressive immunological follicular inflammation starts. Figure 6.4 The eruption is most active over the central back, where the sebaceous activity is at its highest. Figure 6.5 Note that the skin between the folliculopapules is totally normal—no dry itchy scaling, asteatotic eczema, or atopic or contact dermatitis. Figure 6.6 The pattern is folliculopustular on this central and lateral chest. Figure 6.7 The pattern has become folliculopustular on this left shoulder, and the itch is manifested as early excoriations. Figure 6.8 The typical folliculopapules over the right shoulder and clavicle are often ignored, or misdiagnosed as bacterial, leading to antibiotics that make matters worse. Figure 6.9 Same patient as 6.8. The extension of the atopic dermatitis combined with the small folliculopapules from the neck and the forehead into the scalp, with no evidence of psoriatic scale, suggest the combination diagnosis. The failure of oral antibiotics and topical steroid scalp lotions and creams solidifies the case. Figure 6.10 Distal onycholysis points toward psoriasis; itch and response to ketoconazole point to the inciting microbiological stimulus to trauma and Koebnerization. Figure 6.11 Psoriasis descending the back of the neck, with folliculopapules and folliculopustules on the upper back. So far, there has been no adaptive or physiologically important role assigned to the Malassezia organisms. We know that “M. globosa uses eight different types of lipase, along with three phospholipases, to break down the oils on the scalp” [11], but it is not suggested that the species has evolved as a grooming aid for humans. It is more likely that this important human pathogen has evolved with an adaptive capacity that ensures its survival on hosts no matter what different types of lipids it encounters on different skin surfaces. Even more important to the pathogenesis of acne, these lipases and phospholipases are added to those produced in the follicular duct by P. acnes, further increasing the irritation in the duct by the fatty acids produced by the breakdown of sebum triglycerides. It has been speculated for decades that the fatty acids produced by these lipases actually threaten the integrity of the lipid-containing duct wall. Whether the lipases and phospholipases are themselves a threat to the duct wall remains to be seen. Several components of Malassezia with the ability to induce immunoglobulin E have been defined and characterized to the point of sequencing over the past 20 years, and clinical experience confirms a strong association of the yeast and pruritus. Itch is one of the most intractable aspects of atopic dermatitis, but it is also part of the symptomatology of seborrheic dermatitis, seborrhea capitis, some tinea (pityriasis) versicolor cases, and Malassezia folliculitis. Specific treatment provides welcome relief, but persistence is required. As Faergemann has written, a single dose of 400 mg ketoconazole orally every month is effective [12]. Importantly, itch is present in about 25% of cases of acne rosacea and in about the same proportion of acne vulgaris, particularly on the face [13]. What causes this intolerable itch? I strongly suspect but cannot prove that l’acné excoriée des jeunes filles (Section 3.4.6) is in reality acne vulgaris colonized with Malassezia, to which the patient is allergic. Indeed, most acne patients who are drawn to mindlessly (or mindfully) manipulating their lesions likely do so as a result of attention being drawn to the lesions by itch. If history taking includes an inquiry into previous vulvovaginal yeast infections, a negative history is decidedly rare, and a single episode “a long time ago” is the most common story by far. I hear the story daily and suspect that earlier exposure to the antigens in Candida, the better known yeast, is the “sensitizing dose” that leaves the patient with an immune system primed to recognize the Malassezia invading the follicles in both acne vulgaris and acne rosacea. In 1988, Leeming, Holland, and Cunliffe published what I consider to be the most significant and the single most ignored paper in all the work ever done on acne, “The Microbial Colonization of Inflamed Acne Vulgaris Lesions” [14]. Did the misspelling of Cunliffe’s name as Cuncliffe [sic] lead to the paper being missed in literature searches, or was it simply not recognized as a seminal piece of work? Whatever the reason, this paper has languished in almost complete obscurity. The study was simple and elegant. Punch biopsies (3 mm) from the upper back of acne patients with 1-day-old and 3-day-old papules were carefully examined microscopically and cultured. This revealed that biopsies of 3-day-old acne papules hosted a 68% colonization rate by Malassezia furfur. “None [of the patients] had received antimicrobial treatment for at least 4 weeks” [14], but details of earlier treatment are not provided. In addition to the 68% yeast count, “Propionibacterium acnes … constituted a colonizing population in only 71% of papules and was completely absent in 20% of papules” [14]. Two explanations suggest themselves. First, perhaps previous courses of antibiotics selectively sterilized 20% of the pores of detectable P. acnes; or, second, the suggestion that P. acnes is necessary for the production of a simple acne papule may need to be reconsidered. As the authors opine, “The isolation of papules which were not colonized by micro-organisms is at variance with hypotheses stating that inflammation in acne vulgaris is invariably initiated by microbial activity” [14]. It would appear that sterile follicles can indeed produce both non-inflammatory and inflammatory papules. Of note, this does support the anoxia/hypoxia/HIF-1 hypothesis (see Section 7.3) and certainly calls into question the role of P. acnes as the prime mover in acnegenesis. Perhaps a follicle swollen by hormonal overstimulation could leak and release intrafollicular materials (such as the keratin fragments found in acne inversa infiltrates) into the neighboring tissues, stimulating inflammation? This possibility, championed in Section 3.3, was not missed by these authors, who wrote, “Our results suggest that other components of comedones, such as keratins and lipids, should also be considered as potential inflammatory initiators” [14]. That possibility is further considered in this chapter. Acne rosacea’s flare by Malassezia has been all but ignored in the literature, yet I recognize it as a factor two or three times a month. There is no evidence that Malassezia plays any role in acne inversa. That may be partly due to the destruction of the sebaceous glands (see Figure 3.2) and the subsequent lack of sebum to attract the yeast. We live in a sea of microorganisms. Positive cultures as used in modern clinical medicine are more often than not an attempt to confirm the obvious, and to satisfy the community standard of care. Truly obsessive searches for our commensal bacterial friends and potential enemies (now called our microbiome) have demonstrated hundreds and indeed thousands of species living on and in us [15]. So, it is no surprise when a patient who has been on antibiotics, often in a less-than-optimal dose or following an incomplete regimen, then develops a secondary infection with a “new” or resistant organism. If one kills off all the “easy-to-kill” germs, that leaves the “hard-to-kill” ones behind. And if one kills off all the resident bacteria, any of the millions of women who have suffered the pruritic (itchy) tortures of vulvovaginal candidiasis can tell you that the yeast will be delighted to take over both the space and the unused nutrients left by the departed bacteria. Far too many women know all about this. Given the vast population of organisms, all the cultures looking for staph and strep and Gram-negative organisms are going to be positive for something. But is the bacterial organism that is found really the organism responsible, or is it just a local survivor? Certainly, you can eliminate pretty much all common bacteria if you choose the nuclear option (broad-spectrum antibiotics), but are you really helping clear the cause of acne? If you ignore the fact that you are inducing a growing population of Malassezia, and ignore the inflammation that this yeast has triggered, and ignore the vicious, destructive, and self-perpetuating immunological fires that have been set alight, the problem will persist. If not appropriately treated, Malassezia will disappear only when the sebaceous glands are gone (as happens in AI/HS) or when there is no more lipid for the Malassezia to feed upon (as with isotretinoin therapy). When the stimuli or antigens fueling the immune systems are gone, only then will the fires burn out. A far better option from the patients’ point of view is the early elimination of the yeast. Learn how to do this in Section 8.5.3. Mixed in with the entire bacterial and yeasty flora (the flowers), there is really only one little bit of fauna (an animal, but a very small one). Meet the rather amazingly well-adapted little pore mites called Demodex folliculorum. They are cousins of scabies, the cause of probably the itchiest rash you can suffer. Demodex can also be really itchy, and because the mites are active on your skin at night, you may wake up in the morning with scratches on your face you never knew you caused. While Demodex is mostly a problem with acne rosacea, it can be a problem with acne vulgaris as well, but it plays no known role in HS/AI. The tight acroinfundibulum and the lack of sebum ensure this. The Demodex mites normally live head down in the pore, enjoying your sebum (skin oil) as food during the day. The males back out of the pores at night and wander on your skin in search of a mate, then return to the pore before you wake up to shower them off. There may be several in a pore at various stages of development, from eggs to larvae to juveniles to adults. Only if the patient becomes allergic to the mites do they cause any difficulty. They cause redness, swelling, itching, and often little, tiny, easily broken pustules (Figure 6.12). This is the easiest place to find Demodex, either alive before treatment is initiated or after treatment when the dead ones are being pushed out of the pores. Figure 6.12 Two questions must be asked at every visit: “What is in the pustules at this point?” And “What will be needed to get rid of them?”
Follicular flora, fauna, and fuzz
6.1 Propionibacterium acnes (P. acnes)
6.1.1 Normal role of P. acnes
6.1.2 Pathogenic role of P. acnes
6.2 Malassezia species
6.2.1 Normal role
6.2.2 Immunogenicity
6.2.3 Pruritogenicity
6.2.4 Malassezia in the acnes
6.3 Staph, strep, and gram-negative organisms
6.4 Demodex
Follicular flora, fauna, and fuzz
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree






