23 Focal axillary hyperhidrosis
Summary and Key Features
• Sweating is controlled by the sympathetic nervous system; however, eccrine glands are activated by acetylcholine
• Eccrine glands in patients with primary hyperhidrosis do not demonstrate any histopathologic changes or glandular hyperplasia
• Hyperhidrosis prevalence is reported to be 2.8%, though it may be underestimated owing to undiagnosed cases
• The hyperhidrosis disease severity scale (HDSS) is a validated scale that is used in selecting patients appropriate for therapy and assessing effectiveness of treatment
• The starch iodine test is a simple, colormetric test to detect the presence of active sweat glands and identify the surface area involved. In the event of a negative or equivocal result, the hair-bearing skin is a good estimate of the area necessary to treat
• The axillary procedure is well tolerated; no anesthesia is required as pain is minimal
• OnabotulinumtoxinA is FDA approved for treatment of axillary hyperhidrosis. When injected at the depth of the deep dermis and subcutaneous tissue, the chemodenervation is localized, reversible, and long lasting
• Currently the US standard dose listed in the package insert for onabotulinumtoxinA is 50 units per axilla
• An average of 10–15 injections (0.1–0.2 mL each) should be delivered to each axilla
• If symptoms persist after treatment, repeat the starch iodine test to detect any area that may have been missed. A small area of active sweating is easily touched up with 1–2 injections
• If treatment response is overall inadequate or short lived (e.g. 1–2 months), simply increase the dose of onabotulinumtoxinA to 100 units per axilla
• Retreatment with onabotulinumtoxinA will average every 6–7 months, but can vary depending on the patient’s response
Introduction
Hyperhidrosis
Hyperhidrosis may be classified as generalized or focal. Generalized hyperhidrosis typically occurs as the result of an underlying cause (secondary origin). Focal or localized hyperhidrosis may have a secondary origin; for example, lesions or tumors of the central or peripheral nervous system. However, more commonly, focal hyperhidrosis is idiopathic (primary origin) and referred to simply as ‘hyperhidrosis’ (HH). Diagnostic criteria for primary hyperhidrosis have been suggested by a consensus panel (Box 23.1). Evaluation and testing should be tailored by the history and review of systems. This chapter will focus on primary focal axillary hyperhidrosis, henceforth identified simply as axillary hyperhidrosis (AHH).
Epidemiology
The prevalence of HH was reported by Strutton et al to be 2.8%, though it may be underestimated due to undiagnosed cases. The prevalence is similar for men and women, although interestingly, Lear and colleagues reported that women are more likely to seek evaluation and treatment. Trigger factors include emotional stress, higher environmental temperatures, and stimulants such as caffeine. However, episodes of excessive sweating can occur without any known trigger. The body areas most often affected are the axilla, palms, soles, craniofacial, inframammary and groin (Table 23.1).
Table 23.1 Hyperhidrosis site prevalence in a patient population reported with respect to each site*
| Anatomical site | Prevalence (%) |
|---|---|
| Axilla | 73 |
| Palms | 45.9 |
| Soles | 41.1 |
| Face / scalp | 22.8 |
| Groin | 9.3 |
| Other sites (eg. Inframammary, Buttocks, etc.) | 9.6 |
* Data from Lear W, Kessler E, Solish N, et al 2007 An epidemiological study of hyperhidrosis. Dermatologic Surgery 33:S69–S75.
Quality of life
The hyperhidrosis disease severity scale (HDSS) is a validated scale that is used in selecting patients appropriate for therapy and assessing effectiveness of treatment. The HDSS is based on one question the patient can answer in the office: ‘Which best describes the impact of sweating on your daily activity?’ The answer is rated with a single value 1–4, with 3–4 corresponding to uncontrolled severe HH (Table 23.2). It is frequently used in clinical practice to assess the need for therapy and response to it.
Table 23.2 Hyperhidrosis disease severity scale question:
Which best describes the impact of sweating on your daily activity?
| Score | Answer |
|---|---|
| 1 | My (underarm) sweating is never noticeable and never interferes with my daily activity. |
| 2 | My (underarm) sweating is tolerable but sometimes interferes with my daily activity. |
| 3 | My (underarm) sweating is barely tolerable and frequently interferes with my daily activity. |
| 4 | My (underarm) sweating is intolerable and always interferes with my daily activity. |
Clinical assessment of hyperhidrosis
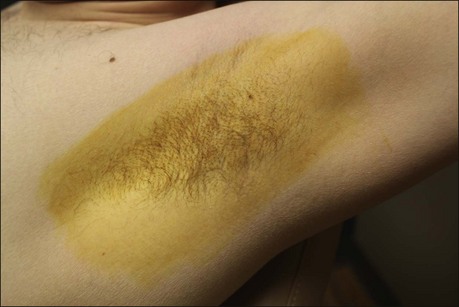
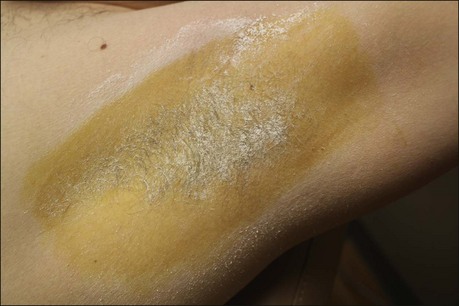
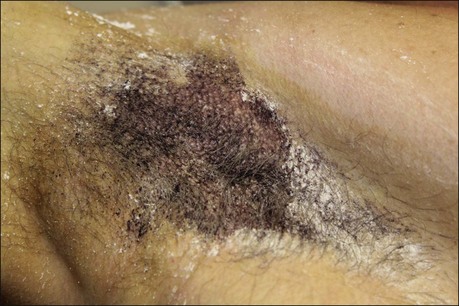
The starch iodine test is a simple way to detect the presence of sweat and identify the surface area involved, however it is not quantitative. The test is effective on both shaved and non-shaved skin. To perform, the skin to be tested is completely dried using a towel. An iodine solution (commonly Betadine®) is painted over the field and allowed to dry (Fig. 23.1). After the solution is thoroughly dry, a starch powder such as plain cooking corn starch is sprinkled on the surface. Various tools may be used to accomplish the distribution of starch such as loose gauze, cosmetic brushes or a fine-opening shaker (Fig. 23.2). Accurate colormetric results are achieved when the amount of powder is minimized. Moisture from sweat dissolves the iodine and starch followed by a chemical reaction producing a purple to black color. True positive reactions have a speckled appearance as moisture is released from duct openings (Fig. 23.3). For iodine-sensitive patients, Alizarin or Ponceau red dye and starch can be used instead. Regardless of which is used, a colorimetric outline of the sweating area is achieved.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree