Fig. 10.1
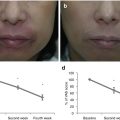
The clinical features of AD vary depending on patient age and duration of disease. In infancy and childhood, AD is characterized by eczematous changes accompanied by strong itching. In adulthood, AD manifests as chronic dermatitis (also called disseminated neurodermatitis) with skin lesions appearing at the sites where itching first occurred (Reprinted from ref. [82] with permission of Japanese Society of Allergology)
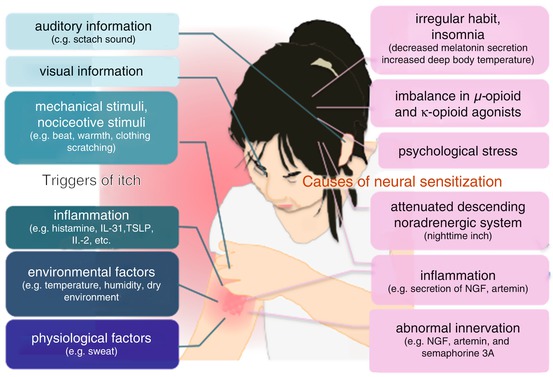
Fig. 10.2
Illustration of triggers of itch and causes of skin hypersensitivity (Reprinted from ref. [82] with permission of Japanese Society of Allergology)
10.2 Neural Transmission and Processing of AD-Related Itching
Electrical impulses traveling through peripheral nerve fibers transmit itching-related signals to the brain, which produces a bodily reaction. Recent studies using animal models of AD indicate that astrogliosis in the dorsal horn of the spinal cord contributes to chronic itching and that the itching sensation is processed in the central nervous system [6]. Although the transmission and processing of AD-related itching in humans requires further clarification, the general process of the neural transmission of itch is summarized here.
Thermal and mechanical stimuli activate receptors on free nerve endings in the skin, which leads to the opening of ion channels and the transmission of electrical current to the spinal cord. Several different itching-related ligands and receptors have been described in the literature (Table 10.1). In particular, itch ligands (i.e., pruritogens) can induce an itching sensation by activating the histaminergic transient receptor potential cation channel subfamily V member 1 (TRPV1) and the non-histaminergic transient receptor potential cation channel member A1 (TRPA1) [7–10], and inhibition of TRPV1 or TRPA1 reduces itching in AD-like animal models [11, 12]. Thus, TRPV1 and TRPA1 have recently received much attention as possible targets for drug development [13]. After itching-related electrical impulses are sent to the spinal cord, they are modulated by interneurons in the spinal cord [14–16] that release inhibitory neurotransmitters such as gamma-aminobutyric acid, glycine, and dynorphins, which reduce the intensity of itch-related signals [15, 16]. Therefore, the control of inhibitory interneurons is another strong candidate approach for treating chronic itching. After processing in the spinal cord, itch-related signals are sent through ascending tracts to the amygdala via the thalamus or medulla oblongata, where they are analyzed and converted into information about their location, strength, and quality [17, 18]. Although the brain circuitry underlying itching is not completely understood, activation of sympathetic neurons in the medulla or midbrain induces the release of noradrenaline, which suppresses itch-related signals through the α(2)-adrenoceptor [19, 20] [19, 21]. Hence, itching is attenuated under conditions of significant sympathetic nervous system activity, such as during the daytime or when individuals concentrate on stimuli other than the itch. However, the central processing of itch-related signals differs between AD patients and healthy individuals [22]. Thus, a greater understanding of the antipruritic nervous system will help advance approaches to treating AD-related itching.
Table 10.1
Major itch-related ligands and receptors
Ligand | Receptor | Ref. |
|---|---|---|
Histamine | Histamine receptors | [61] |
Kallikreins, tryptase, endogenous/exogenous proteases | Protease-activated receptor 2 | |
Bradykinin | Bradykinin receptors | [64] |
Serotonin (5-HT) | 5-HT receptor | [65] |
Endothelin-1 | Endothelin receptors | |
Interleukin (IL)-31 | IL-31 receptor | [30] |
Thymic stromal lymphopoietin (TSLP) | TSLP receptor | [29] |
Substance P | Neurokinin-1 receptor | |
Platelet-activating factor (PAF) | PAF receptor | [70] |
Leukotriene B4 (LTB4) | LTB4 receptor | [71] |
Electrophiles, oxidants, pro-inflammatory agents | TRPA1 | |
12-HPETE | TRPV1 | [74] |
Artemin | GDNF family receptor α3 | [27] |
IL-2 | IL-2 receptor | [75] |
Gastrin-releasing peptide (GRP) | GRP receptor | |
β-endorphin | μ-opioid receptor | |
Acetylcholine | Acetylcholine receptor | [80] |
Calcitonin gene-related peptide (CGRP) | CGRP receptor | [81] |
10.3 Inflammation, Dry Skin, and Itching
In skin conditions such as eczema and dryness, molecules that cause itching (i.e., pruritogens), including cytokines and chemical messengers, are released from the affected area and act on nerve endings in the skin [23, 24] (Table 10.1). The itching caused by pruritogens triggers scratching of the affected area [24], which aggravates the dermatitis, resulting in a vicious “itch-scratch cycle.” Dryness and inflammation, such as that which occurs in AD, induce elongation of sensory nerves in the epidermis under the stratum corneum, which causes skin hyperesthesia [25]. This aberrant nerve elongation in AD involves nerve growth factor, artemin, interleukin (IL)-31, and semaphorin 3A [26–28]. Histamines derived from the degranulation of mast cells and substance P released from nerve endings can act directly or indirectly on elongated nerves, causing itching. The inflammatory mediators thymic stromal lymphopoietin and IL-31, which are derived from epidermal keratinocytes and infiltrated lymphocytes, respectively, cause itching through direct actions on nerve fibers [29, 30]. Furthermore, elevated levels of autotaxins (e.g., lysophospholipase) due to cholestasis induce itching in hepatic disorders [31], and blood autotaxin concentration is correlated with itch intensity in AD patients [32].
10.4 Temperature and Itching
Healthy individuals can experience relief from histamine-evoked itching by exposure to extreme temperatures [22, 33, 34], such as taking a very hot shower or using ice to cool the itchy area. However, although AD-related itching can be suppressed by painful cold stimulation, it is worsened by painful heat stimulation [22, 33, 35]. Moreover, AD patients often report that their itchiness increases in warmer conditions [5, 36]. Thus, AD may involve abnormal hyperesthesia, causing patients to feel thermal stimulation as an itching sensation. Unfortunately, this heat-provoked itching responds poorly to treatment [36], and although it is thought to result from the sensitization of peripheral or central nerves, its underlying mechanism is still uncertain.
Peripheral nerve fibers are distributed across the dermis and are affected by factors secreted by surrounding fibroblasts. To identify molecules that contribute to skin nerve fiber hypersensitization, we comprehensively analyzed the expression of genes in dermal fibroblasts that were stimulated by allergic inflammation- and itch-related factors. We found that substance P induced the expression of artemin, a neurotrophic factor that is important for the development of sympathetic innervation, in dermal fibroblasts [27]. Whereas no artemin expression was detected in normal human skin, we found its accumulation in dermal lesions associated with AD and nummular eczema [27]. Furthermore, we observed that mice treated with artemin on their back exhibited increased sensory innervation of the skin and abnormal behavior such as rubbing their skin across their entire body when exposed to a warm temperature (38 °C), which was not observed in artemin receptor knockout mice. This finding suggests that the abnormal local accumulation of artemin in the skin causes systemic thermally provoked itching, although the underlying mechanism is unclear. However, we found that capsazepine, a selective antagonist of TRPV1, did not suppress the artemin-induced abnormal behavior, indicating that the systemic response occurs independently of TRPV1 [27].
In our clinical practice, we have encountered AD patients who developed itching immediately after taking a hot bath or removing their clothes, which we assume arises from a rapid change in the temperature of the skin surface. This phenomenon has been confirmed by experimental studies. For instance, Pfab and colleagues observed increased itching when histamine-treated skin was exposed to rapidly alternating cool and warm temperatures (between 25 and 32 °C) [37]. Therefore, even within temperature ranges typically encountered in daily life, rapid changes in temperature can intensify itch. Therefore, patients with AD should be careful to avoid extreme temperatures in their daily life, such as hot bathwater or cold air conditioning. Japanese guidelines for AD recommend that the temperature of bathwater should be set between 38 and 40 °C [38].
10.5 Perspiration and Itching
Mammals are homeothermic organisms that maintain a stable body temperature through thermal control mechanisms such as perspiration, expiration, and heat transfer [39]. In humans, perspiration through eccrine sweat glands distributed throughout the body is an important physiological function that regulates body temperature [39].
Sweat contains several molecules that are beneficial for the skin, such as natural moisture-retaining factors (e.g., urea, sodium lactate), bactericidal peptides (e.g., dermcidin, cathelicidin, β-defensin), and secretory IgA to defend against infection [40]. Sweat also inhibits cysteine and serine protease activity, helps prevent inflammatory responses to allergens, and aids in the formation of mature stratum corneum [41–44]. Although sweat can reduce the intensity of experimentally induced itching in healthy individuals [45], many patients with AD report that sweating aggravates itching [46]. In one study, basophils derived from AD patients showed positive reactions in a histamine release test to semipurified antigens extracted from the sweat of healthy subjects, suggesting the existence of a “sweat allergy” [47]. However, another study failed to detect positive reactions in a longer-term sweat patch test [48]. Thus, sweat may provoke an acute, but not a delayed, allergic reaction. Interestingly, an antigen derived from Malassezia globosa, a fungus indigenous to the skin, was identified in the sweat of AD patients and may be involved in this “sweat allergy” [49]. In addition, reduced levels of antimicrobial components in sweat are associated with a weaker ability to defend against infection in AD patients [40].
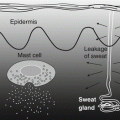
Symptoms of AD can be aggravated by leaving excess sweat on the skin. One cause of this aggravation is that excess sweat increases the pH level of the skin surface [40]. Under normal conditions, the pH of sweat remains low because sodium bicarbonate (HCO3 −) and alkali ions in sweat are reduced via reabsorption by sweat ducts [39]. However, excess sweat on the skin leads to a failure of HCO3 − reabsorption, which increases the pH of the skin and thereby promotes detachment of the horny cell layer and increases susceptibility to inflammation [40]. In addition to increasing pH, various molecules contained in sweat (e.g., Malassezia-derived antigens) can cause itching. Moreover, the beneficial effects of sweat, such as proteinase inhibition, may be lost when excess sweat is left on the skin. Hence, AD patients are advised to wash or wipe off excess sweat with a wet towel in cases of itching accompanying perspiration.
AD patients show less ability to perspire, either due to abnormalities in the production of sweat or the obstruction of its release to the skin surface [40]. One possible mechanism by which the release of sweat is obstructed is the formation of horny plugs in sweat pores [50], although it is not clear whether this might be due to inflammation-related keratosis or dryness resulting from reduced sweating. Also, Shiohara and colleagues detected the presence of dermcidin, an antimicrobial peptide produced by sweat glands, surrounding sweat ducts in lesioned area of AD patients [51], suggesting the leakage of sweat outside of sweat ducts, which might reduce the amount of sweat discharged to the skin surface and increase heat retention. Perspiration tests using acetylcholine or temperature loading have confirmed that sweat secretion is reduced in AD patients [51–54], which is related to dysautonomia and a tendency toward an anxiogenic personality [53]. Furthermore, we recently discovered that histamine suppresses perspiration by affecting sweat glands [55], suggesting that allergic inflammation is a cause of reduced sweating in AD patients.
Because sweat aggravates AD, patients may be told to avoid sweating, although there is no evidence that this improves skin symptoms [56]. Rather, as proper treatment of skin symptoms in AD also restores perspiration function, increased sweating to facilitate heat transfer should be considered an important goal in the management of AD.
10.6 Daily Rhythms and Itching
AD patients often complain that their itching increases at night. One potential explanation is that body temperature declines overnight and fluctuates during sleep. The drops in body temperature are accompanied by dilation of peripheral vessels, which elevates skin temperature and may increase itching [57]. Another possible explanation is that transepidermal water loss increases at night, making the skin susceptible to itching. Nighttime is also associated with decreased cortisol and increased IL-2 production, which are related to inflammation [57]. Furthermore, staying up late reduces melatonin production by the pineal gland, which inhibits the normal decline in body temperature, and circadian dysregulation can cause an imbalance in agonists of μ-opioid and κ-opioid receptors, which may increase the severity of itching [57]. Therefore, AD patients are advised to practice good sleep habits and maintain regular sleep schedules.
10.7 Psychological Factors and Itching
Itching can arise not only from stimulation of the skin but also from visual or auditory information. For example, a person may have an urge to scratch himself or herself upon seeing an image suggestive of itching, such as being bitten by a mosquito, or hearing the sound of another person scratching their skin [58, 59]. This phenomenon, known as “contagious itch” [60], is stronger in AD patients than in healthy individuals [60]. The onset and worsening of itching can also be caused by psychological factors and related conditions such as insomnia.
Conclusion
The management of AD is continuing to improve through advances in our understanding of factors that exacerbate itching, their mechanisms, and their countermeasures. Because AD can be aggravated by commonplace features of our environment within a normal physiological range, it can be difficult to provide sound guidance for minimizing the symptoms of AD. As some questions remain unresolved, we hope that evidence accumulating from recent and future studies can be applied to improve the health and quality of life of patients with AD.
Conflict of Interest
The authors have nothing to declare.
References
1.
Hill LW, Sulzberger MB. Evolution of atopic dermatitis. Arch Dermatol Syphilol. 1935;32(3):451–63.Crossref
2.
Brocq L, Jacquet L. Notes pour servir a l’histories des néurodermatitis: du lichen circumscriptus des anciens auteurs, ou lichen simplex chronique de M. le Dr. E Vidal. Ann Dermatol Syphiligr. 1891;97:193–208.