Fig. 14.1
Facial preadipocytes are more responsive to rosiglitazone-containing differentiation compositions, relative to abdominal preadipocytes. Human facial and abdominal preadipocytes in control media (CD, insulin, dexamethasone, and IBMX), or CD plus 10 μM rosiglitazone (CDR). Microscopic images of the cells were taken on day 14 after plating. The pictures are representative of three individual experiments. DEX dexamethasone, INS insulin
The ability to differentiate was maintained through multiple passages longer in facial preadipocytes than abdominal preadipocytes (Fig. 14.2). It was determined that facial preadipocytes may be induced to differentiate at a passage greater than 10. The morphological and molecular characteristics at passage 11 were similar to those after the third passage (Fig. 14.3a). In Fig. 14.3b, quantitative polymerase chain reaction (qPCR) analysis demonstrated that the expression of the major adipocyte markers: fatty acid binding protein 4 (FABP4), PPAR-γ, and fatty acid synthase (FAS; glucose transporter 4 (GLUT4), G-protein coupled receptor 81 (GPR81), leptin, and adiponectin as well but not shown) are similarly and significantly induced during differentiation of both facial and abdominal preadipocytes. The late-passage (passage 11) facial preadipocytes did not have significantly different expression levels of the adipogenic or the lipolytic genes tested (more than 20 genes), and these expression levels were similar to those of differentiated early-passage (passage 3–5) abdominal cells.

Fig. 14.2
Facial preadipocytes retain their ability to differentiate through later subpassages than abdominal preadipocytes. Human facial and abdominal preadipocytes at the indicated subpassages were induced to differentiate by CDR. Images of the cells were taken on day 14 after plating. The pictures are representative of three individual experiments. CDR control differentiation media + 10 µM rosiglitazone, P passage number
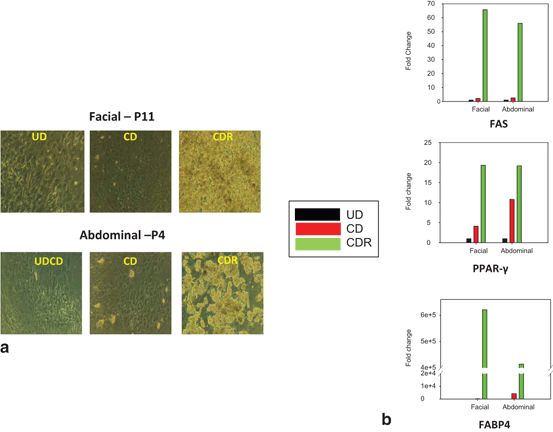
Fig. 14.3
Morphology and expression of lipid gene markers in facial and abdominal preadipocytes: effect of passage number. Facial and abdominal preadipocytes were grown for 14 days in the CD media or CDR media. a Images are representative of four individual experiments. b Gene expression was evaluated by qPCR and normalized by expression of the RPL13A gene. UD undifferentiated (cultured in growth medium), CD control differentiation, CDR CD + 10 µM rosiglitazone, FABP4 fatty acid binding protein 4, FAS fatty acid synthase, P passage number, PPAR-γ peroxisome proliferator-activated receptor gamma, qPCR quantitative polymerase chain reaction
On day 14 or 15 after adding the differentiation media, preadipocyte cultures became fully differentiated adipocytes, as determined by morphology and adipocyte marker gene expression (FABP4, GLUT4, and PPAR-γ). To test the functional response of these cells, passage 3–5 facial and abdominal preadipocytes were treated with 2 µM of isoproterenol, a β2-adrenergic receptor agonist (Fig. 14.4). After 5 h, lipolytic rate measured by glycerol release was twofold greater in abdominal than facial adipocytes (Fig. 14.4a). After 1 week of exposure to isoproterenol, facial preadipocytes retained more lipid droplets than abdominally derived cells (Fig. 14.4b). Histological examination revealed more lipid droplets of greater diameter in the facial cells than in the abdominal adipocytes.
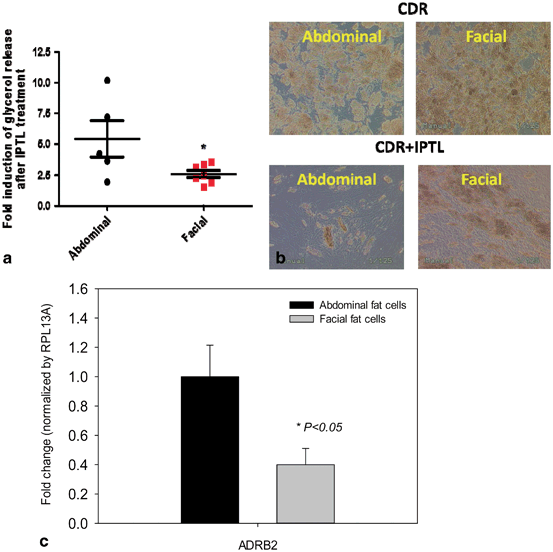
Fig. 14.4
β-adrenergic receptor expression and responsiveness to isoproterenol in differentiated facial and abdominal preadipocytes. a Acute glycerol release after 5 h isoproterenol exposure (2 μM). Data is represented as mean ± SEM. Abdominal cells, n = 5 donors and facial cells, n = 7 donors. *Student t test P < 0.05. b Facial and abdominal adipocytes images before and after 1 week of isoproterenol treatment. c After 1 week of isoproterenol exposure, the expression of lipid processing genes was evaluated by qPCR analysis and was normalized by expression of the RPL13A gene. Abdominal cells, n = 6 donors and facial cells, n = 4 donors. CD control differentiation, CDR CD + 10 µM rosiglitazone, ADRB2 β2-adrenergic receptor
An array of genes associated with lipolysis was then tested to examine the possible differences in expression levels between facial and abdominal cells (Fig. 14.4c). Among the 25 genes tested, the expression of the β2-adrenergic receptor (ADRB2) was significantly different between facial and the abdominal adipocytes. The lower expression levels of ADRB2 from facial adipocytes compared with abdominal adipocytes was consistent with our finding of reduced lipolytic rate in facial fat cells (Fig. 14.4a), suggesting the underlying mechanism.
The differences noted above in expression of the β2-adrenergic receptors in differentiated preadipocytes from abdominal and facial sources were identified using cultured adipocytes. Further the differences in gene expression observed between these tissue sites also existed in vivo. The gene profiles using tissue obtained from age-matched individuals (45–60 years old) undergoing either facelift or abdominoplasty demonstrated that most gene markers were expressed at similar levels in tissues from both sites. As shown in Fig. 14.5a, significantly less expression of FAS, CPT2, and GLUT4 in facial versus abdominal adipose tissue was observed. Although β2-adrenergic receptor expression levels was lower in facial versus abdominal adipose tissue, the difference was not statistically significant. The expression the β3-adrenergic receptor was detected in abdominal adipose tissue, but was completely undetectable in the facial adipose tissue (Fig. 14.5b).
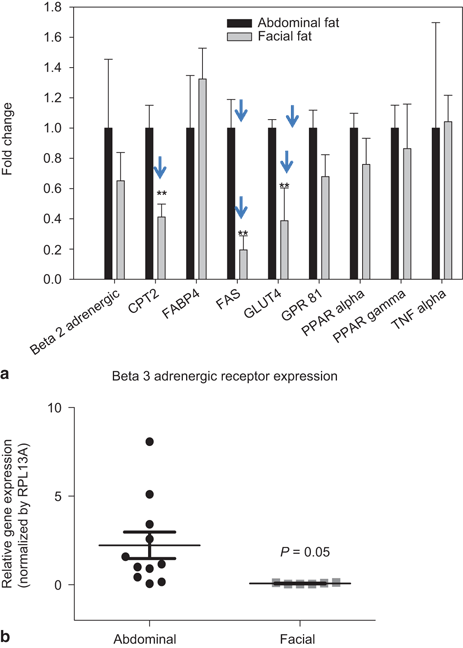
Fig. 14.5
Expression of adipocyte markers in facial and abdominal subcutaneous fat tissues. mRNA was isolated immediately following tissue harvest from females 50–60 years of age. Gene expression was evaluated by qPCR analysis; expression of fat cell markers was normalized by expression of the RPL13A gene (mean ± SEM). a Lipid metabolic gene expression in subcutaneous fat tissues (n = 4 donors per group), b β3-adrenergic receptor expression in facial (n = 5 donors) and abdominal (n = 10 donors) tissues. *P < 0.05 versus abdominal cells. BLD below limit of detection, CPT2 carnitine palmitoyltransferase 2, FABP4 fatty acid binding protein 4, FAS fatty acid synthase, GLUT4 glucose transporter 4, GPR81 G-protein coupled receptor 8, PPAR-α peroxisome proliferator-activated receptor alpha, PPAR-γ peroxisome proliferator-activated receptor gamma, TNF-α tumor necrosis factor alpha
Microarray analysis identified unknown genes that were regulated depot specifically; in five human abdominal adipocytes and four facial. Depot specific patterns of gene expression were observed by principle component analysis (PCA), abdominal samples were separated by facial samples in general, based on the overall expression profiles (Fig. 14.6a). Total of 283 probes differentially regulated between the two depots were identified (Fig. 14.6b). Around 126 probe sets were significantly upregulated in abdominal fat as compared to facial fat, with fold change not less than 2 and the overall FDR less than 0.05. Using the same criteria, 157 probe sets were significantly downregulated in abdominal fat as compared to facial fat (Fig. 14.6b). The detail expression profiles of these 283 probe sets are also illustrated in this heat map Fig. 14.6b. The lists of the top ten genes regulated differentially were summarized in Table 14.1. Interestingly, the majority of upregulated genes in abdominal compared to facial adipocytes, in other words, significantly downregulated in facial were the HOX transcription factors, which have also been identified in other depot comparisons; for example, abdominal versus gluteal regions (Gehrke et al. 2013).
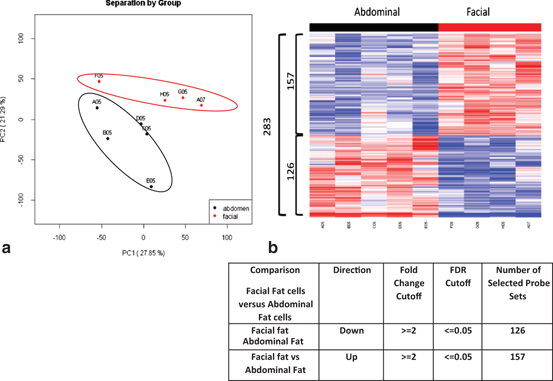
Fig. 14.6




Depot specific gene expression profile and comparison in human facial versus abdominal fat cells by Microarray analysis. a PCA analysis demonstrated a distinct gene expression pattern between two depots (Abdominal fat cells; n = 5 donors, Facial fat cells; n = 4 donors). b Heat-map illustration showing identified 283 probes differentially regulated between the two depots. Around 126 probe sets were significantly downregulated in facial fat cells as compared to abdominal fat cells. Additionally, 157 probe sets were significantly upregulated in facial fat cells as compared to abdominal fat cells
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree