Facial Reanimation in the Oncologic Patient Using Nerve Grafts and Nerve Transfers
Joseph H. Dayan
DEFINITION
Facial reanimation in the setting of parotid tumors can be challenging, sometimes with unpredictable functional and aesthetic outcomes.
Additionally, patients with locally advanced disease may not be ideal for multiple stage procedures over the course of several years.
A rapid and reliable functional result with the least number of procedures becomes a priority.
This chapter focuses on reanimation strategies mainly using nerve grafts and nerve transfers depending on the clinical scenario.
Dynamic panfacial reanimation is the goal.
The reanimation procedures presented in this chapter are ideally performed at the time of tumor ablation if possible.
ANATOMY
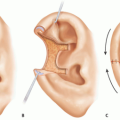
The masseter muscle is one of the muscles of mastication.
It arises from the lower border and medial surface of the zygomatic arch and inserts into the lateral surface of the mandible (FIG 1).
The muscle consists of three layers—superficial, middle, and deep.
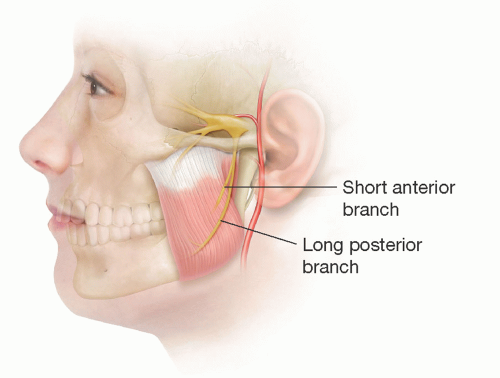
The superficial muscle fibers pass backward and downward; the deeper fibers are more vertical. The motor nerve to the masseter is derived from the mandibular division of the trigeminal nerve. It leaves the infratemporal fossa by passing over the lateral pterygoid muscle and mandibular notch. It enters the masseter muscle from its deep surface and courses down between its deep and middle layers by having a diameter of 1 to 2 mm. It becomes more superficial distally toward the anterior inferior part of the muscle and runs alongside the (friable) vascular pedicle that arises from the inferior alveolar artery.
The facial nerve exits the stylomastoid foramen, turns superficially, and immediately enters the substance of the parotid gland where it is classically described as dividing into five branches—temporal (frontal), zygomatic, buccal, mandibular, and cervical.
The nerve usually divides into upper (temporal) and lower (cervical) trunks at first, from which these branches arise. In practice, they constitute five variably interconnected groups of branches hereafter referred to as divisions.
Despite the various studies of facial nerve anatomy, it may be more useful to envision the facial nerve as consisting of physiological and functional divisions that do not necessarily correlate to the anatomic distribution of branches but instead relate to muscular groups and actions.
The temporal branch of the facial nerve exits from the upper pole of the parotid gland at the level of the zygomatic arch and becomes superficial being plastered onto the underside of the temporoparietal fascia.
The course of the nerve follows a line drawn from 0.5 cm below the tragus to 1.5 cm above the eyebrow, but 3D modeling demonstrates the branches cover a triangular area bordered between a line through the palpable zygomaticofrontal and zygomaticotemporal sutures and a vertical line 1 cm in front of the auditory meatus.
At the lateral margin of the frontalis, these branches run deep to frontalis and orbicularis oculi (which it innervates), eventually reaching the glabella region to supply the depressors of the brow.
The zygomatic division leaves the parotid gland to run just inferior the zygomatic arch immediately deep to the superficial musculoaponeurotic system (SMAS) to innervate the elevators of the upper lip and lower portion of orbicularis oculi.
Distally, it runs on the undersurface of the zygomaticus muscle complex before ramifying more medially in the face. The buccal branches exit the anterior border of the parotid gland and run inferior to the parotid duct and on the surface of the buccal fat pad deep to the SMAS layer to supply the muscles of the mouth and cheek.
Both zygomatic and buccal divisions have a highly overlapping distribution of innervation that allows transection of one or more branches without weakness; this is the basis of cross-facial nerve grafting.
The mandibular branch leaves the lower portion of the parotid gland and swings down below the ramus of the mandible to supply the muscles of the lip and chin.
Cadaveric dissections demonstrate that the nerve always comprises at least three major branches that run on the undersurface of the platysma and superficial to the facial vessels.
PATIENT HISTORY AND PHYSICAL FINDINGS
Unlike the nononcologic patient population, many of these patients receive adjuvant radiotherapy, with resulting volume loss and tissue contracture, and potentially negative effects on neurotization.
While smile is often the most visually impressive feature to reanimate, the patient’s blink is the most time-sensitive and important function in terms of daily living.
Once the patient has been denervated for over 2 years and the orbicularis oculi has atrophied beyond salvage, it becomes very challenging to recreate a blink.
In contrast, a smile can be reliably restored years after irreversible denervation with a free gracilis or temporalis transfer.
This is also the reason the author generally reserves temporalis transfer for cases in which native facial musculature is unsalvageable, as the temporalis generally does not restore eye closure.
Lower lip motion and depression complete a symmetric outcome.
If the patient presents within 2.5 years after the initial denervation, a needle electromyography (EMG) is performed specifically to assess viability of the orbicularis oculi, zygomaticus major, and depressor anguli oris.1
If there is muscle viability, nerve-to-masseter and minihypoglossal transfers are typically performed; if there is no viable muscle, functioning muscle transfer is required.
SURGICAL MANAGEMENT
The first choice in nerve reconstruction of a discreet segmental defect is always primary nerve grafting. Only the facial nerve can provide spontaneous facial motion.
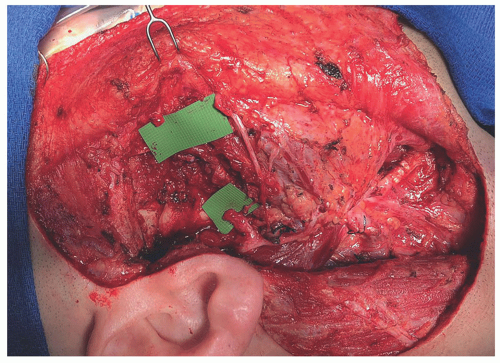
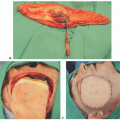
The ideal patient is one that has a relatively short segmental defect, no tumor in the proximal facial nerve trunk, and no prior radiation (FIG 2).
An anterolateral thigh free flap provides not only soft tissue replacement to fill the defect but also donor nerve graft in the form of a motor branch to the vastus lateralis muscle. The nerve can be left attached to the pedicle as a vascularized nerve graft or, more commonly, harvested separately to allow for greater freedom of inset.
Patients who do not have a reliable proximal facial nerve stump available can have smile and at least lower eyelid motion restored with a nerve-to-masseter transfer. This will provide volitional but not spontaneous and emotional motion of the zygomaticus major and orbicularis oculi complex in 3 or 4 months.
While not ideal, nerve-to-masseter transfer is highly reliable, and some patients may not require an upper eyelid weight and frequent eye drops once reinnervation is complete.
In cases in which there is no proximal facial nerve trunk available, the author’s practice has evolved to using dual nerve transfers so as to avoid a grotesque mass facial motion from a single nerve transfer to all distal branches.
The patient is evaluated preoperatively to confirm normally functioning masseteric and hypoglossal nerves. If there is a deficiency, these nerve donors are avoided, and other donors such as the spinal accessory nerve can be considered.
Cross-face nerve grafting provides the potential for spontaneous motion when the ipsilateral facial nerve is unavailable.
One significant drawback of this technique is the variability in strength of the outcome compared with nerve transfers that deliver reliable power but cannot compete with cross-face nerve grafting in terms of spontaneity.
It is also most commonly performed in two stages, which may not be feasible in cases of more advanced disease.
For patients in whom the ipsilateral nerve is unusable, however, this is the only procedure that provides spontaneous motion.
TECHNIQUES
▪ Nerve Grafting and Direct Repair
Nerve Reconstruction
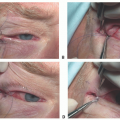
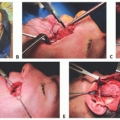
Following nerve harvest, the nerve is carefully prepared by removing the adventitia (TECH FIG 1A).
In theory, this maximizes the likelihood for nerve graft take and survival in the way a thinner skin graft has higher survivability than a thicker composite graft (TECH FIG 1B).
The nerve is then brought to the field, and a neurorrhaphy is performed from the inferior fascicles of the upper division of the facial nerve to the distal zygomatic facial nerve branch for smile (TECH FIG 1C).
An epineurial repair is performed with 10-0 nylon suture.
Ideally, during the time of tumor ablation, the specific fascicular bundles from the proximal facial nerve are stimulated and mapped to match the distal remnant facial nerve branches targeting smile and blink.
Following proximal neurorrhaphy, the nerve graft is divided to allow adequate length for tension-free nerve repair (TECH FIG 1D).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree