Kofi Boahene, Patrick Byrne, and Barry Schaitkin address questions for discussion and debate:
- 1.
What forms of nonsurgical therapy (physical therapy, electrical stimulation, and so forth) do you recommend to improve the outcome of facial paralysis and why?
- 2.
Explain your preoperative assessment tool for deciding what to do (Eye reanimation? Who needs a medial canthoplasty? and so forth).
- 3.
How do you assess the results of management of facial paralysis?
- 4.
Discuss the use of end-to-side anastomosis (Viterbo concept of something for nothing). Should it be used; why or why not?
- 5.
What is your preferred method for temporalis muscle transposition and why? Are there any tricks to improving the results?
- 6.
Do you use cross-facial nerve jump grafts and use them for free muscle innervation? If so, what are the pearls you have learned from this technique and when do you use it?
- 7.
Analysis: Over the past 5 years, how has your approach evolved or what have you learned/observed in working with reanimation?
Kofi Boahene, Patrick Byrne, and Barry M. Schaitkin address questions for discussion and debate:
- 1.
- 2.
- 3.
How do you assess the results of management of facial paralysis?
- 4.
- 5.
- 6.
- 7.
What forms of nonsurgical therapy (physical therapy, electrical stimulation, and so forth) do you recommend to improve the outcome of facial paralysis and why?
Boahene
To date, the best reanimation surgeries have fallen far short of completely restoring the complex expressive movements and function characteristic of the normal unparalyzed face. This is partly because changes in the somatotopic arrangement that occur in the facial nucleus and the facial motor cortex cannot be directly corrected with reanimation surgery. To influence the inaccessible aspects of the facial neuromuscular network, nonsurgical therapies are needed. Various forms of nonsurgical therapy and intervention have been shown to minimize the effects of aberrant facial nerve regeneration, improve facial symmetry, help patients adapt a new social smile, and maximize the effectiveness of any reinnervated facial muscle or substitute muscle. Facial neuromuscular retraining, speech therapy, and the selective use of chemodenervation agents are the main nonsurgical interventions I recommend for facial paralysis.
Facial neuromuscular rehabilitation (fNMR) or mime therapy was introduced in 1980 in the Netherlands, specifically for patients with facial nerve paralysis, through collaborative work between mime actors and clinicians. Neuromuscular retraining unlinks undesired motions from desired ones using slow, small-amplitude, desired motions while consciously suppressing the undesired ones. As the undesired activity is suppressed, the range of the primary movement gradually extends, increasing excursion, strength, and motor control. Surface electromyographic (EMG) feedback, mirror feedback, and video biofeedback are essential complementary tools that help bring desired movements to conscious control. Although there is a paucity of well-designed, randomized controlled trials on the effectiveness of facial exercises on the functional outcome of facial paralysis, selected publications support its beneficial role. Pereira and colleagues performed a systematic review and meta-analysis of 132 studies that investigated the role of facial exercises in facial paralysis and concluded that it was effective. Beurskens and Heymans in a randomized controlled trial concluded that mime therapy improved facial symmetry and reduced the severity of facial paralysis.
My facial reanimation patients see a physical therapist before any intervention who specializes in fNMR. I also recommend early facial retraining exercises to Bell palsy patients to minimize the severity of any synkinesis that may occur. A study by Nakamura and colleagues showed that biofeedback works better for prevention of synkinesis as opposed to treatment of synkinesis. Due to the intense efforts needed to achieve visible improvement in their synkinesis, patients often fail to reach their desired goal because of the difficulty of maintaining motivation during training. Initiating biofeedback techniques soon after a facial injury motivates patients to prevent rather than treat synkinesis.
Once patients have become comfortable with their self-directed exercises, I often aid their progress with selective chemodenervation with botulinum toxin injection. The selective use of botulinum toxin helps uncouple facial muscle groups involved in synkinesis. I treat both the paralyzed and unparalyzed face to produce balance and symmetry. Patients with lip incontinence, masticatory difficulties, and articulation changes undergo speech therapy. A speech therapist measures interlabial pressures and provides exercises that aid with lip seal. In selected cases, I use injectable fillers to aid with lip continence.
Facial exercise therapy is also essential in the acquisition and adaption of a temporal smile after a temporalis tendon transfer procedure. The main goal of temporal smile therapy is to transfer upper lip excursion in smile function to the transposed temporalis muscle. There are 3 main phases in the therapy involved in acquiring the temporal smile:
- 1.
The first phase, the mandibular phase, involves mobilizing the mandible to contract the transferred temporalis muscle to elevate the oral commissure.
- 2.
The second phase, the voluntary temporal smile, replaces the mandibular phase and involves contraction of the temporalis muscle without movement of the mandible.
- 3.
The third phase, the spontaneous temporal smile, concentrates on adapting the voluntary temporal smile as the expressive smile for social settings.
Patients with facial paralysis are increasingly inquiring about the role of acupuncture in the treatment of facial paralysis. I do not recommend for or against the adjunctive pursuit of acupuncture but caution my patients from delaying definitive care when nerve grafting is recommended. In addition, I caution my patients concerning the potential for direct nerve injury from the acupuncture needle when repaired nerves are superficial.
Byrne
I encourage physical therapy. Although functional electrical stimulation has a body of supportive literature for spinal cord injury, I do not believe this is the case for facial rehabilitation. The data for targeted facial retraining via traditional physical therapy—with or without biofeedback—are not conclusive either. There is a logical and neurophysiologic basis, however, for encouraging this. We work with a physical therapist who has a particular interest in facial retraining. The goals are 2-fold, depending on the nature of the paralysis:
- 1.
The first goal is to encourage purposeful and appropriate movements.
- 2.
An equally important second goal for many (most) patients is to limit synkinesis.
Schaitkin
I use physical therapy extensively in the rehabilitation of nonsurgical and surgical patients who have had facial paralysis. I have done so since 1991. Initially I referred patients to Richard Balliet and colleagues. Balliet used the phrase, “neuromuscular retraining of facial paralysis,” referring to combining patient education in basic facial anatomy, physiology, and kinesiology; relaxation training; sensory stimulation; EMG biofeedback; voluntary facial exercises with mirror feedback; and spontaneously elicited facial movements. Most recently I have been working with Todd Henklemann, using his physical therapy techniques. I have been impressed with the ability of these techniques to improve scores using the Ross-Fradet grading system. Much of this work owes it origins and proliferation to Jackie Diels. Her concepts of incorporating surface EMG biofeedback with a comprehensive rehabilitation strategy have solid foundation and are used effectively by therapists throughout the country. This is not a single modality approach to facial paralysis patients.
When evaluating these techniques, patients need to be separated into those who have an intact nerve after and are recovering from a viral facial paralysis and those who have had interruption of the nerve and nerve grafting, substitution, or other non-neural reanimation techniques. For the viral facial paralysis patients, I see no need to send patients who are in excellent prognostic groups: incomplete paralysis, excellent evoked electrical testing in the first 10 days, or early onset of recovery after complete paralysis (<4 weeks). I have seen benefits on multiple levels for sending patients who have are not in these groups. Early therapy is aimed at patients who have weakness without synkinesis.
Facial nerve physical therapy is often done in concert with the use of botulinum toxin. The Cochrane Collaboration in 2008 published an article, “Physical Therapy for Bell’s Palsy” (Idiopathic Facial Paralysis) (Review). They selected randomized and quasi-randomized controlled studies involving physical therapy. Their conclusion was that a review of available literature involved a wide variety of physical therapy techniques used for treating Bell palsy. They found a “lack of high quality evidence to support the use of these strategies.” It is my impression, however, that the use of physical therapy for these patients is not controversial (a statement that may, in and of itself, be controversial). The problem with this review is, that even the selected studies it is based on, begin therapy at a variety of times post-insult, making comparisons difficult. Patients not only benefit from the coaching and emotional support of a therapist, but also have documented recovery.
I find the area of electrical stimulation much more controversial, however. Although there are animal studies that suggest that electrical stimulation may have a positive effect in that it shortens the early stage of recovery after rodent facial nerve crush injury, I have not seen it beneficial in my patient population. The vast majority of patients who are seen in my practice after receiving electrical stimulation seem to follow the natural history of the disease, and those who are at the end of the natural history recovery have not shown improvement with additional electrical stimulation. Referring back to the Cochrane article regarding electrical stimulation, “almost all the outcomes reported failed to show any statistically significant difference between electro-therapy or exercises and conventional or no treatment.” Some of the results both in animals and humans have shown worse results in the electrical stimulation group.
Facial reanimation patients need physical therapy for the following reasons: their facial paralysis is much longer than in viral patients and extends while they wait for nerve growth to occur; they have emotional needs from a facial nerve and sometimes diagnostic point of view; and their best possible outcome, an House-Brackmann (HB) grade III, in the case of simple nerve repair, is considered a poor outcome from a viral facial paralysis standpoint. Patients with nerve substitutions, such as hypoglossal to facial jump grafts, and those with innervations of free muscle transfers must relearn their smile and they greatly benefit from facial retraining with experts who dedicate their practice to the care of facial paralysis patients. Hadlock and colleagues recently described a mixed group of reanimation patients, 111 of whom were sent for physical therapy. Of these, 83% reported subjective improvement and 97% had objective changes using a grading system.
Explain your preoperative assessment tool for deciding what to do (Eye reanimation? Who needs a medial canthoplasty? and so forth)
Boahene
My preoperative assessment tool for facial reanimation focuses on determining and documenting the cause of the paralysis, the reversibility of the paralysis, the functional deficits that are present, and the specific goals of patients seeking reanimation. An accurate and thorough preoperative assessment is essential in selecting appropriate reanimation methods and helps prioritize the intervention.
Determining the cause of facial paralysis
The causes of facial paralysis vary widely but can be categorized into
- •
Idiopathic facial paralysis (Bell palsy)
- •
Paralysis resulting from tumors (facial neuroma, acoustic neuroma, geniculate hemangioma, parotid neoplasms, and so forth)
- •
Developmental or traumatic facial paralysis.
Establishing the cause of the facial paralysis gives essential information about the prognosis and expected course of the paralysis. A thorough history is usually adequate to determine the cause of facial paralysis. Adult patients presenting with long-standing paralysis may not have precise information, however, about the etiology of the paralysis. For example, patients with paralysis secondary to birth trauma or early-childhood infection may confuse their acquired paralysis secondary to birth trauma for a developmental paralysis. When necessary, we obtain a high-resolution MRI and temporal bone CT scan to evaluate the intracranial and extracranial course of the facial nerve and to evaluate for skeletal and soft tissue abnormalities. Even after a thorough history and high-resolution imaging studies, the cause of a facial paralysis may remain elusive and a diagnostic surgical exploration may be necessary. Occult neoplasms can masquerade as Bell palsy, which delays the timely diagnosis and treatment of the neoplasm and the resulting facial paralysis. We obtain and document information about the duration and progression of the paralysis and the functional deficits present and a standardized quality-of-life assessment is obtained.
Establishing if the paralysis is reversible
When evaluating patients for potential facial reanimation, it is important to establish whether the paralysis is reversible or irreversible. The reversibility of the paralysis depends on several factors, including
- •
Duration
- •
Degree of injury (complete vs partial)
- •
Status of the facial muscles and facial nerve.
A reversibly paralyzed facial muscle has physiologically viable muscle fibers with intact neuromuscular junctions that respond to ingrowing axons. Reinnervating such a muscle results in a functional and contracting muscle that can restore tone and movement to the face. On the contrary, atrophic and fibrotic muscles (irreversible paralysis) have both mechanical and physiologic barriers to incoming axons and do not respond to reinnervation. In irreversible facial paralysis, options that rely on recruiting a new source of functional muscle to replace the damaged facial muscles are required for facial reanimation.
The duration of facial paralysis is an approximate predictor of the reversibility of the facial paralysis. The returns on nerve grafting after 1 year of paralysis diminish significantly. When the facial nerve is known to be anatomically continues, nerve grafting after 2 years is possible and has been reported. An EMG is helpful in providing objective support for the reversibility of the facial paralysis. I obtain an EMG on all patients who are considered for facial reinnervation procedures. The presence of persistent electrical silence with attempted voluntary movement on EMG signifies irreversible paralysis and in such patients we consider transfer of regional or free functional muscle. When the EMG reveals fibrillation potentials, then the paralyzed facial muscle is a viable end organ and options for nerve grafting, depending on the duration of paralysis, are considered.
Assessment of functional and aesthetic deficits from facial paralysis
Paralysis of the facial muscles results in functional and aesthetic changes that affect eye protection, breathing, smiling, lip continence, and speech. Compensatory changes on the contralateral unparalyzed side also contribute to the overall morbidity of facial paralysis. Faulty regeneration of repaired or spontaneously recovering nerve may result in sequelae that may be worse than the facial paralysis. In planning for facial reanimation, I assess functional and aesthetic changes by a subunit approach (ie, the upper face [brows and eyelids], midface [smile, lip levators, nasal patency, and lip continence], lower face [lip depressors], and neck [platysma muscle]). In addition, I assess for global facial response to facial paralysis and nerve regeneration, including signs of aberrant reinnervation (ie, hypercontraction, hypokinesis, and synkinesis).
Assessment and reanimation of the upper face
Brow ptosis
The ptotic brow resulting after facial paralysis produces heaviness along the temporal brow and may obstruct the peripheral visual field. The visual field obstruction is usually more pronounced in elderly patients. In younger patients, the aesthetic asymmetry from brow ptosis is usually more bothersome than visual field obstruction. Patients are asked to frown and raise their brow to document the dynamic changes in the frontalis muscle. By manually elevating the temporal brow, we determine the degree to which brow ptosis contributes to visual field obstruction and select the best vector for brow pexy if necessary. Endoscopic brow suspension for female patients and younger patients is our main approach to correcting paralytic brow ptosis. In elderly patients who are aesthetically less sensitive, we find the open midforehead or a temporal brow lift satisfactory. The emphasis on brow suspension in the paralyzed forehead is placed on the temporal portion of the brow because that portion tends to droop the most. We use selective chemodenervation of the contralateral frontalis muscle to help achieve forehead and brow symmetry. I personally do not favor the routine use of cross-facial nerve grafting for reanimating the paralyzed brow although it has been successfully demonstrated. This is due to the need for harvesting a sural nerve graft, scarring from exploring both sides of the face, and risk of injury to the intact contralateral facial nerve.
Eyelids
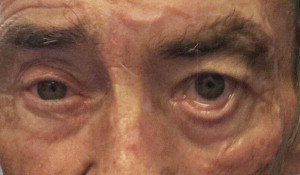
Paralysis of the orbicularis oculi muscle is associated with significant dysfunction that can lead to keratitis, corneal abrasion and loss of vision. Restoring eyelid function and corneal protection remains one of the primary focuses of our reanimation efforts in facial paralysis. Our preoperative assessment of the paralyzed eyelid systematically documents the ability of patients to voluntarily and involuntarily blink, corneal sensation, globe position (positive vs negative vector globe), medial and lateral canthal laxity, position of lower lacrimal punctum, tearing, and degree of scleral show. Terzis and Kyere have published a grading system for eyelid paralysis, which we have found helpful. The eyelid examination is documented by video and photographic capture. With the head in a neutral position, voluntary and involuntary blinking is recorded. Patients are then asked to close their eye gently and then tightly. The presence or absence of twitching, spasm, or synkinetic movement of the lips and neck muscles are noted. Patients who are considered for nerve grafting undergo preoperative EMG. We address the upper and lower eyelids separately with either static procedures, regional muscle transfer, or nerve grafting procedures based on patient age, degree of lagophthalmos, ectropion, and EMG findings.
Table 1 shows the indications and various techniques we use in addressing the paralyzed eyelid. In younger patients with reversible paralysis of the orbicularis oculi muscle, we strongly consider reinnervating the muscle with nerve grafts either by direct muscle nerotization or interpositional grafting if the nerve branch to the muscle can be identified. When considering upper eyelid loading, we use the platinum chain implants weighing between 0.8 g and 1.2 g. Heavier weights often result in excessive mechanical ptosis that may obstruct vision and result in an unacceptable eyelid asymmetry. We recommend upper eyelid loading more for its ability to aid in voluntary blinking than for its gravity effects. Patients with a negative vector eyes where the globe is anteriorly positioned are not good candidates for upper eyelid loading. This is because the implant sits over the protruding globe and the resulting inertia makes it less effective in initiating eyelid movement. In patients with thin upper eyelid skin, we recommend placing a barrier over fascia or allograft barrier over the implant to minimize extrusion. The lower eyelid position and function is arguable more important in corneal protection in the upper eyelid. The essential functional features of the lower eyelid are its vertical position, pumping effect on the lacrimal canaliculi, apposition of the lacrimal puncta to the globe, and the medial and lateral canthal support.
| Anatomic Site | Procedure | Indication |
|---|---|---|
| Brow | Unilateral endoscopic brow lift Temporal brow lift Midforehead brow lift | Paralytic brow ptosis, temporal brow hooding, peripheral visual field obstruction from ptotic brow Limited to elderly men with deep forehead rhytids |
| Upper eyelid | Upper eyelid loading with platinum chain implant. Fascia or allograft barrier is placed over the implant in thin skin eyelids Minitemporalis muscle transfer Levator advancement or plication | Poor blink, 2–3 mm lagophthalmos, favorable globe position Poor corneal sensation, absence Bell phenomenon, poor blink, negative vector globe Blepharoptosis secondary to aberrant facial nerve regeneration |
| Lower eyelid | Lower eyelid vertical suspension with a spacer graft Lower eyelid fascial sling suspension | Ectropion with >2–3 mm scleral show Ectropion, patient with medial and lateral canthal tendon laxity, lacrimal punctum eversion |
| Medial canthopexy. Perform through a postcaruncular incision with canthal supetention to periorbital posterior-to-posterior lacrimal crest | Medial canthal laxity, medial eyelid margin eversion, lacrimal punctum displaced away from globe, lacrimal punctum can be distractly laterally past the medial limbus | |
| Lateral canthopexy Tarsal strip, canthal tightening Direct neurotization of orbicularis oculi muscle (jump graft from ipsilateral facial nerve stump or hypoglossal nerve) | Lateral canthal laxity, used in combination with medial canthopexy Extreme canthal laxity in elderly patients Presence of fibrillation potential or reduced motor activity on EMG, resected or unidentifiable ipsilateral facial nerve branch to orbicularis, young or middle-aged patient | |
| Upper lip | Direct nerve repair with end-to-end coaptation Interpositional grafting Transposition of intratemporal facial nerve to hypoglossal nerve with end-to-side anatomosis with 30% partial hypoglossal neurotomy Hypoglossal to facial nerve jump grafting Hyoglossal to facial nerve transfer Cross-facial nerve grafting | Lower facial hypokinesis, asymmetry, distorted smile |
| Lower lip | Direct repair or with cable graft when proximal and distal ends of the marginal nerve branch can be identified Transfer of marginal branch to hypoglossal nerve for end-to-side coaptation Direct neurotization of depressor labia muscle Diagastric tendon transfer | Asymmetric lower lip depression during smile with EMG evidence for a reversible paralysis Asymmetric smile with EMG evidence of irreversible paralysis When the proximal facial nerve in unavailable but a long marginal branch reaches the hypoglossal nerve When distal facial nerve unavailable but facial muscle are viable Delayed repair of isolated lower lip paralysis |
| Neck | Platysma resection Platysma and SMAS suspension | Synkinesis with platysma hyper contraction Correction of paralytic jowling and platysma banding |
Table 1 shows indications and the technique we commonly use to address the paralyzed lower eyelid. As a general principle, we favor dynamic reanimation of the lower eyelid over static procedures. Among the static eyelid procedures available, we place more emphasis on procedures that maintain or improve the vertical height of the lower eyelid because loss of tone in the paralyzed orbicularis oculi muscle results in eyelid ectropion. We reserve lid-shortening procedures to elderly patients who have excessive canthal laxity. Although lateral canthal tightening is commonly described, laxity of the medial canthus is often overlooked. Our main indications for medial canthopexy are eversion of the medial lower eyelid margin, distraction of the lacrimal punctum away from the globe, and the ability to distract the lower eyelid lacrimal punctum lateral to the medial limbus ( Fig. 1 ). Faulty regeneration of the facial nerve innervating the upper eyelid muscle can result in obstructive blepharospasm that appears as blepharoptosis. This ptosis temporarily responds for chemodenervation and also to levator aponeurosis advancement for a more long-term correction.
Assessment and reanimation of the paralyzed midface
Dynamic reanimation of the paralyzed midface aims to restore upper lip elevation and midfacial tone and symmetry at rest. In addition, midfacial reanimation has the potential to improve nasal breathing in cases of paralytic nasal obstruction and improve vertical support of the paralyzed lower eyelid. Improving tone in the midface and lip seal also helps with speech and lip incontinence. In assessing the midface for smile restoration, the pattern of smile based on movement on the contralateral side is determined. Specifically, we determine the predominant vector of movement during upper lip excursion. In tendon or muscle transfer procedures, we try to reproduce this movement by inserting the transferred muscle along this vector. Patients are asked to produce a gentle smile followed by an exaggerated full smile while being video recorded. A ruler is position next to the oral commissure of the nonparalyzed side to serve as a scale for measuring the degree of excursion off videos. We also evaluate the function of the masseter and temporalis muscles. When surgical procedures have previously been performed around the temporalis muscle, a muscle stimulator is useful in determining contraction of the temporalis muscle. We clinically determine and document function of cranial nerves V, IX, and XII as potential motor sources for nerve grafting. Nasal examination is performed to document static and dynamic valve collapse on the paralyzed side. Lip continence and speech assessment are performed by a speech pathologist and measurements of interlabial pressures from the midlips and lateral lips are obtained.
Assessment and reanimation of the lower face
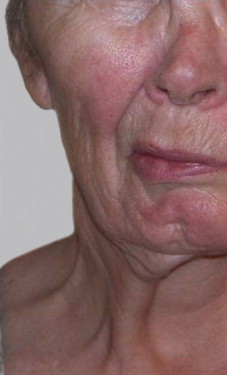
Lower lip
The lower lip is essential in sealing the lip for lip continence to prevent drooling and in the generation of plosive sounds during speech. The depressors of the lower lip also contribute to the production of a full smile that reveals both the upper and lower lip. Isolated injury to the marginal branch of the facial nerve is common and manifests in a distorted smile ( Fig. 2 ). Lower lip function before reanimation is assessed with video documentation. Patients are asked to smile with a gentle and an exaggerated smile to determine the contribution of the lower lip depressors to their natural smile. Interlabial pressures are measured to objectively document differences between the paralyzed and normal sides of the lip. In patients who have recovered from previous facial paralysis, it is essential to document the presence of synkinetic movement with other facial muscles as well as the effects of any platysma hypercontracture on lip movement. The degree of lip atrophy is documented to support the need for lip augmentation. Table 1 shows procedure use for lower lip reanimation.
Neck
The changes in the neck after facial paralysis are mainly seen in the platysma muscle and may be negligible or severe. The platysma muscle is often the recipient of excessive innervation after facial nerve injury with spontaneous recovery and after nerve repair. This manifests as hypercontracture of the platysma muscle that produces a tight feeling in the neck. The platsyma muscle extends over the mandible and continues with the submusculoapaneurotic system (SMAS) system of the midface. As a depressor of the lower face, hypercontraction on the platysma muscle is a major antagonist to lip elevation and can impair movement of the upper lip when smiling. Resection of the hypercontracting platysma muscle or temporary chemodenervation relieves the facial tightness and may allow better excursion of the lip levators. In assessing for platysma resection, patients are asked to smile broadly and presence and location of tight platysma band are marked. The excursion of the oral commissure is recorded on video before and after division of the platysma muscles. We commonly perform platysma muscle resection under local anesthesia through small 1-cm incisions.
Byrne
This is a lengthy process for me. An initial new patient consult is routinely scheduled for 1 hour—and we use that time up. We discuss the functional aspects of their facial nerve disorder (most importantly, eye protection, but also often speech, oral competence, and nasal breathing) as well as the psychosocial aspects. The psychosocial impact of the facial paralysis is more important to most patients. I believe that we have an obligation during the preoperative assessment to educate patients about symmetry and how faces are perceived by observers. It is a complex topic, but the patients who comprehend this find that it reli is empowering.
To grossly simplify, the social aspect of facial paralysis is a problem of perceived asymmetry; because all faces have some degree of asymmetry, observers routinely ignore this in daily life. With facial paralysis, suddenly the degree of asymmetry is such that it triggers conscious recognition on the part of the observer. Thus, the reality is that the problem is not the dysfunction but the degree of asymmetry that is produced and whether or not this is enough for others to routinely notice. This is our great opportunity for helping patients. We analyze the entire face—upper third, middle third, and lower third—and systematically seek ways to improve 2 things: (1) symmetry) and (2) attractiveness. These are inextricably linked.
As for specific decisions, there are a few personal preferences. I engage patients in personal banter early in the consultation and try to elicit laughter, observing all the while their own particular adaptive and maladaptive facial movement patterns. I believe that for most patients with brow asymmetry significant enough to warrant intervention, a bilateral endoscopic brow lift is the best choice. There are exceptions. Not all patients need anything done to their lids. Many need nothing surgical. A simple rule to assess risk of exposure keratopathy that I heard years ago from Dr Seiff at UCSF is using the acronym, BAD:
B = Bell phenomemen—those without are at greater risk
A = Anesthesia of the cornea—also a greater risk patient
D = Dry eye history
Many patients benefit from upper lid loading, and we prefer the platinum chain ( Fig. 3 ). (For the lower lid, if lateral ectropion is present [particularly, if symptomatically relieved by the application of gentle digital pressure supporting the lower lid, mimicking a canthopexy], then I usually prefer the lateral transorbital canthopexy, as described by Kris Moe and colleagues [ Fig. 4 ]). This is a procedure that is reversible, adjustable, and preserves native anatomy well without shortening the horizontal aperture. It is not ideal for older patients in whom the horizontal dimension of the lid is expanded, however. Those patients tend to require horizontal shortening, such as with a tarsal strip procedure. Medial ectropion requires a specific medial canthopexy. Lid retraction is treated when clinically significant. This is a tough problem, particularly in patients with a negative vector ( Fig. 5 ).










