Steven Dayan, David Ellis, and Mary Lynn Moran address questions about Facial Fillers for discussion and debate:
- 1.
Are there different indications for the different fillers?
- 2.
In your opinion, what are the durations of the various fillers?
- 3.
Is there still a role for the use of collagen injections?
- 4.
What complications concern you and what do you do in your practice to attempt to avoid or minimize these?
- 5.
What type of anesthesia do you use, when, and why?
- 6.
Analysis: over the past 5 years, how have you modified your techniques or approach or what is the most important thing you have learned/observed in working with injectables and fillers?
Steven H. Dayan, David Ellis, and Mary Lynn Moran address questions about facial fillers for discussion and debate:
- 1.
Are there different indications for the different fillers?
- 2.
In your opinion, what are the durations of the various fillers?
- 3.
Is there still a role for the use of collagen injections?
- 4.
- 5.
What type of anesthesia do you use, when, and why?
- 6.
Dr Dayan presents a video of his technique for facial fillers using a blunt tip cannula. Available at : http://www.facialplastic.theclinics.com/
Are there different indications for the different fillers?
Dayan
It has been my impression that, as we gain more experience, the greater are the apparent differences between the various fillers approved by the Food and Drug Administration (FDA). Once recognized, each filler’s unique physical properties can be relied on to achieve specific outcomes in individual patients. Furthermore, the fillers are priced differently, have different durations, and are marketed differently. All of these factors ought to be taken into consideration before deciding which filler is best for a patient.
Hyaluronic acid fillers
The most popular fillers are the hyaluronic acids (HAs), as they comprised 85% (1.3 million of the 1.5 million) of the filler treatments in 2010. The 2 leading brands Restylane/Perlane (Medicis Aesthetics, Scottsdale, AZ, USA) and Juvéderm Ultra/Ultraplus (Allergan, Irvine, CA, USA) make up most of the market share, and although they are often thought of interchangeably they have very different physical properties. Juvéderm is positioned as the “smoother filler.” Its gel-like 24 mg/mL concentration with hydrophilic properties 6 times that of Restylane is likely the reason behind its smoother effects. Restylane, a firmer product defined by its rheological properties G′, is more than 6 times more resistant than Juvéderm to deformation.
It is these precise differences between Juvéderm and Restylane that I frequently rely on for specific indications. Juvéderm-corrected areas tend to lead to a fuller and more diffuse augmentation as water is absorbed. I also have noticed that the Juvéderm augmentation may be dynamically related to the body’s current levels of hydration. Anecdotally I have a young staff member who, after receiving Juvéderm into her lips, has a noticeably variable augmentation of her lips the day after she has a meal of salty food. I have not witnessed this with the less hydrophilic Restylane. Consequently, knowledge of its greater hydrophilicity can be used to an advantage, especially in the lips ( Fig. 1 ). For the younger patient who is seeking enlarged lips and already has a full body lip, I will place 0.4 mL of Juvéderm deep into each lip body. For the more mature patient with natively thinner lips and/or who is overly concerned about a recognizable difference, I will place a fine whisper (0.3–0.5 mL) of Restylane within either vermilion border. This procedure has the effect of obviating fine vertical rhytids of the lip and nicely defining the lip border without extending into the cutaneous lip or body. Lipstick can now be placed without concerns of migration, and the lips appear more youthful but not enlarged.
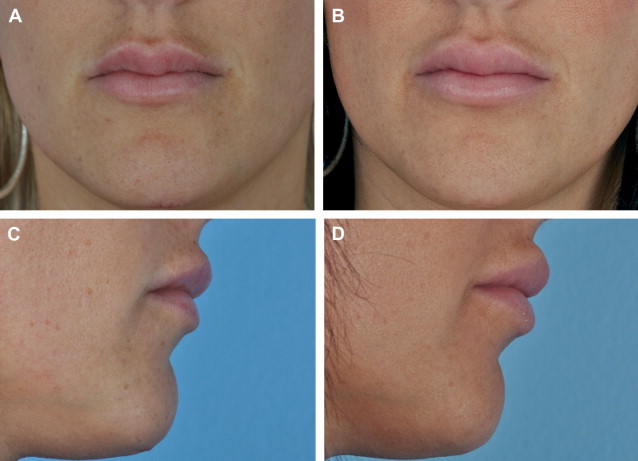
However, because of its firmer nature and less hydrophilic properties, Restylane can lead to lumps and irregularities if not placed evenly. Juvéderm, with its highly hydrophilic nature, tends to attract more fluid and, therefore, results in a diffuse filling effect, which I believe with its nonparticulate gel consistency work to deliver its marketed “smoother” effect. However, if a larger quantity of Juvéderm (more than 1.0 mL approximately) is placed into a lip, it can rather quickly lead to an unnatural, large shelf-like appearance of the cutaneous white lip.
Beyond the lips, the differences in hydrophilic nature of the HAs can also be relied on when correcting or augmenting the nasolabial folds (NLF) and cheeks. For the buccal cheek space, I find Juvéderm’s superior hydrophilic and diffuse filling properties more beneficial, whereas in the infraorbital tear trough I prefer Restylane. Nowhere on the face are the differences between Juvéderm and Restylane more apparent than when placed into the loose areolar tissues surrounding the orbital region. I find Juvéderm to be contraindicated because it will reliably swell, resulting in dark puffy undereye circles representative of a tired unflattering appearance. Although it can occur with both products under the eyes, the Tyndall effect, whereby optical chamber of HA and fluid reflects blue light, is more commonly associated with Juvéderm. In those with thin, translucent skin, the blueness is distractingly obvious. Nowadays I find myself removing more misplaced Juvéderm from under the eyes than insertion of Restylane.
Both products are also manufactured in a more robust version that is intended for deeper placement into the soft tissues with a potential for greater duration. Perlane, at the same concentration (20 mg/mL), is a version of Restylane with larger particle size, and Juvéderm Ultraplus, at the same concentration as Juvéderm, has greater viscosity. However, I remain unconvinced that a clinically recognizable difference is appreciable between the more robust version and its less expensive version by the patient or me. More recently, I have been limiting my use to the Restylane or Juvéderm Ultra versions. However, I eagerly anticipate the arrival of the highly regarded large-particle Voluma and Restylane SubQ for facial shaping. In addition, Belotero (Merz Aesthetics, San Mateo, CA, USA), a smooth-consistency monophasic HA, seems likely to gain FDA approval soon, and is reported to provide correction without risk for a Tyndall effect because of its trademark cohesive polydensified matrix properties. This product may prove to be superior for treating the tear trough.
Calcium hydroxyapatite fillers
Radiesse (Merz Aesthetics, Inc, San Mateo, CA, USA), a biostimulating filler approved by the FDA in 2006, comprises calcium hydroxyapatite (CaHA) microspheres (30%) surrounded by a carboxy methylcellulose resorbable aqueous gel carrier (70%), leaving behind a matrix scaffolding of CaHA beads and setting up a potential framework for neocollagen deposition. Although I do not find the collagen inducting property to be clinically significant, I find CaHA to be a very reliable and safe product. Radiesse’s high G′, indicating the product’s firmness and ability to project tissues, is a major advantage for correcting deeper wrinkles and folds. I find it particularly helpful for treating those with thicker skin and those who desire highlighting or emphasis of bony prominences such as malar, chin, and jawline areas. Its insignificant hydrophilic properties mean “what you see is what you get” after placing it, and any further augmentation is negligible. Therefore, it is important to realize that if not placed thinly or diffusely, it can result in hard streaks and nodules. I prefer to premix 0.3 mL of 1% lidocaine with epinephrine into the product just before treatment. This FDA-approved process not only makes the product more malleable but also reduces discomfort during placement.
In economic terms, most of the fillers are priced competitively; however, Radiesse contains 1.5 mL of product per syringe, compared with 0.8 mL and 1.0 mL for Juvéderm and Restylane, respectively. Although variations in retail pricing to the patient exist across the country, for most practices, Radiesse with the additional product per syringe may be a more economical option. It goes further for the dollar and this may be a deciding factor for some patients. Radiesse is not immediately reversible, whereas the effects from misplaced or excess HA can be reversed within 12 to 24 hours following an injection of hyaluronidase (Vitrase; ISTA Pharmaceuticals, Irvine, CA, USA). This factor may be an influence on the inexperienced filler patient or doctor.
Poly -l -lactic acid
Poly -l -lactic acid (Sculptra; Sanofi Aventis, Bridgewater, NJ, USA) received FDA approval for cosmetic use in 2009. It is a product that relies on its controlled foreign-body biostimulatory properties, and is preferred by many physicians and patients for its subtle and progressive augmentation. However, too robust of a response can be problematic, resulting in nodules and, rarely, granulomas. I target this product for a small niche of patients requesting global facial volumization. I often prefer fat in such situations, but in thin individuals lacking adequate fat stores for harvesting or in human immunodeficiency virus patients with lipodystrophy, I find Sculptra to be the best and most appropriate filler. Although its augmenting benefits are slowly realized, they can be very impressive; but as the results are not immediate, patients’ expectations have to be well managed. In an era of immediate gratification, the necessity to reconstitute with water days in advance and having to do a series of treatments to recognize the benefits make this product less user-friendly than other off-the-shelf fillers.
Silicone and polymethylmethacrylate
Other fillers that I use less often include the permanent silicone (Silikon 1000 purified polydimethylsiloxane; Alcon Laboratories, Fort Worth, TX, USA) and polymethylmethacrylate (PMMA) (Artefill; Suneva, San Diego, CA, USA). I find the hardness properties of these products very advantageous for filling in the fine rhytids of the upper lip, scars, and deep etched-in dermal rhytids. However, silicone’s long-term questionable side effects prevent me from selecting it in younger patients, the lip bodies, or those with thicker skin. Artefill comprises PMMA beads surrounded by a bovine collagen, and in 2006 became the only permanent FDA-approved filler product in the United States. However, it has had difficulty gaining popularity. Its properties are impressive, as is its 5-year safety data. I use this product for deeper etched-in wrinkles of the face in those insistent on a permanent solution.
Ellis
At present, there is no universally accepted classification system for injectable fillers but they can be categorized into the following parameters: type of filler, method of action, and duration of action. Fillers can be either biodegradable or nonbiodegradable. The source of the filler may be natural/animal, synthetic, or natural synthetic. Method of action can be based on replacement of volume or stimulator of fibroplasia and collagen, or both. The duration of action may be temporary, semipermanent, or permanent. Patients are more interested in their duration of the filler in the specific areas they wish to be augmented.
Indications can be viewed as filler-based and patient-based depending on the characteristics of each filler and where the filler is to be injected. For instance, in the tear troughs, I like to use a minimal amount of filler that is not stiff. HA-based fillers work well in this area. Several pitfalls, however, may occur in this area:
- 1.
Too superficial an injection can result in a bluish hue under thin skin, known as the Tyndall effect.
- 2.
Too much volume can result in lumpiness and exacerbation of bags under the eyes, especially when the patient is smiling.
I have used Juvéderm Refine, Juvéderm Ultra Plus with Lidocaine, and Juvéderm Ultra with Lidocaine for this area. Juvéderm Voluma (20 mg/mL total HA concentration) is too viscous and thick to inject in the tear trough, periorbital areas, or lips, but is an ideal product for the cheek and chin. Juvéderm Voluma has been approved for use in Canada but is still under clinical investigation in the United States. As one of the initial clinical investigators of this product in Canada, we have seen tremendous results in patients requiring volume replacement for facial rejuvenation. I find the product easy to inject (although it is noticeably firmer and requires more force to inject than traditional high molecular weight [MW] HAs), to sculpt, and to mold. It is well tolerated by patientsl as evidenced by their satisfaction with the product, likelihood of returning for additional treatment, and their willingness to recommend the product to others. However, I have injected into the lips and the patients have not felt it to be too stiff or firm. In previous years, Gore-Tex is been used to insert the lips for a permanent filler, and Gore-Tex is much firmer than Voluma. Patients enjoy the length of time Voluma lasts.
The added benefit of using an HA-based filler is that it can be dissolved out with hyaluronidase if the patient is unhappy with the product. In areas where inadvertent skin necrosis is a concern, hyaluronidase is essential in one’s armamentarium to treat such a disastrous complication.
Defects to be corrected by fillers as a first choice include the NLF, marionette and oral commissural lines, labiomental crease, mid-cheeks/zygoma, chin, lips, philtrum, ear lobe, tear trough, infrabrow, temporal depressions, and hands. In young Asian patients, we have used fillers to augment the dorsum of the nose, project the nasal tip, and occasionally have placed filler in the nasal spine to allow increased tip rotation. Fillers can also be used for dynamic wrinkles of the upper face and vertical wrinkles of the white lip, but are less effective that other rejuvenation techniques available such as Botox or laser resurfacing. Sites where fillers are not indicated would include nonextensible scars, neck and necklines, and sites of previously implanted permanent or unknown fillers. One exception to this would be our clinical experience of HA fillers in patients with previously implanted Bio-Alcamid (Polymekon, Brindisi, Italy), a nonabsorbable hydropolymer used as injectable permanent filler for cosmetic treatment and soft-tissue reconstructive defects.
Moran
With our enhanced understanding of the role of fillers in panfacial rejuvenation, the indication for fillers has expanded. Even without any significant changes in technology of the fillers themselves, we have a greater appreciation of their varied applications.
I use lighter, less viscous HA products near the surface of the skin and in tear troughs. I use heavier, more viscous products in the deeper subcutaneous layers and to augment fat and bony deficiencies in the face. I often layer them when appropriate.
In your opinion, what are the durations of the various fillers?
Dayan
Duration of the products varies not only between the individual filler types, but also can be highly variable amongst its own brand. The HAs absorb water, gaining additional correction after being injected. HAs are reversible, metabolized via enzymatic degradation and reaction with a reactive oxygen species, and have FDA approval for 12 months on initial treatment with Juvéderm and 18 months with retreatment for Restylane. However, many experienced practitioners acknowledge incidences of correction that have extended well beyond 1 year. In fact, I have many patients in whom fullness and/or visible product is still evident at 3 years or longer. By contrast, I have others in whom it is completely resorbed within 3 months.
The reason some patients retain fillers and others rapidly metabolize it remains elusive. However, I believe a few factors beyond the individual patient’s metabolism contribute to the duration of the product. If placed into a confined nonmobile space, the product seems to last longer. I have many patients in whom I have placed a small quantity, less than 0.2 mL, into the tight space between the thick superficial musculoaponeurotic system and thin periosteum above the bony nasal dorsum, and the correction has persisted beyond 2 years. A 2007 report from the University of Michigan showed that HA can stimulate collagen production, perhaps contributing to its extended correction. In addition, I have seen many patients with worm-like lumps and bumps in the immobile NLF dermis from product placed years prior. I am certain the product is in the dermis because without any bleeding, it is easily expressed after puncturing the skin with an 18-gauge needle. It is interesting that the HA filler looks exactly like it did before being injected.
I also believe that, if placed in large quantities or if an early retreatment is given, the product seems to last longer. In 2010, we published data showing persistence of correction at 36 months with retreatment in the NLF. Although shown not to experience the fibrous ingrowth like the permanent hydrogels, it seems the HAs, with their very thin fibrous capsule, can develop a homeostatic and synergistic relationship with the surrounding tissue exchanging metabolites. Also, perhaps in greater quantities or concentration, the enlarged surface area results in inefficient metabolism of the product. I do not recognize any differences in persistence between Juvéderm and Restylane and routinely tell patients they can expect between 6 months to a year from the product, with it lasting longer in some people and shorter in others.
CaHA is metabolized by enzymatic breakdown with absorption of microspheres evident at 9 months. I find CaHA lasts predictably and routinely from 9 to 12 months, regardless of the patient. However, I did have one patient who was taking ibandronate (Boniva; Roche Therapeutics Inc, Nutley, NJ, USA) and she felt that it dissipated quicker. However, I cannot confirm this nor have I seen any scientific validation.
Poly -l -lactic acid is likely degraded by hydrolysis and extracellular enzymes, and subsequently broken down by macrophages. In my experience, it can be expected to last from 12 to 18 months following a series of 3 treatments. It is difficult, however, to gauge its persistence because the correction is so gradual and diffuse.
Artefill does provide for appreciable permanent results. However, in the NLF, where I have the most experience with the product, it seems necessary to gain complete correction first with multiple syringes, 2.0 to 3.0 mL.
Ellis
Temporary fillers are usually resorbed by the body in about 6 months to 1 year. Semipermanent fillers may last 1 to 2 years. Permanent fillers are basically nonresorbable.
Temporary fillers include:
- 1.
Bovine-derived collagen (Zyderm, Zyplast)
- 2.
Allogenic collagen (Cosmoderm, Cosmoplast, Cymetra, Fascian)
- 3.
Synthetic HA materials (Juvéderm Refine/Ultra/Ultra Plus, Restylane and Perlane, Teosyal, Esthelis, Isogel, Belotero).
Semipermanent fillers are often alloplastic in nature with the exception of Juvéderm Voluma. These fillers include:
Radiesse: an aqueous-based gel carrier with spherical particles of synthetic CaHA
Sculptra: a biodegradable poly- l- lactic acid polymer
BeautiCal: known as Outline in Europe, an absorbable polyacrylamide gel.
Juvéderm Voluma is unique in that it confers all the advantages of HA-based fillers while providing greater longevity. Juvéderm Voluma is a smooth but highly cohesive and viscous gel that uses a combination of high-MW and low-MW HA polymer chains ( Fig. 2 ). The addition of these low-MW chains improves the effectiveness of cross-linking, resulting in a product that is both cohesive and viscous ( Fig. 3 ). In vitro, this translates to a product that is able to retain its structure and resists migration.


At present, Artefill is the only FDA-approved permanent injectable filler for facial soft tissues. Artefill received FDA approval in only 2006 although its predecessors, Arteplast and Artecoll, have been available for over a decade outside the United States. This product consists of PMMA microspheres suspended in 3.5% bovine collagen solution and 0.3% lidocaine.
Liquid silicone was the first highly popularized permanent injectable filler. It is a colorless and odorless product composed of long chains of polymerized dimethylsiloxane. It was well accepted because of its natural feel and ease of injection. It was also well tolerated in small volumes. However, widespread use led to recognition of a fibrotic reaction followed by low-grade inflammation and subsequent capsule formation. Publicized tragedies of organ failure and death from granulomatous hepatitis, pulmonary embolism, and silicone pneumonitis led to the withdrawal of silicone by the FDA in 1991. At present, silicone oil is approved by the FDA only for ophthalmic use.
Polyalkylimide hydrogels have also been marketed as permanent fillers, the most popular being Bio-Alcamid. Bio-Alcamid is a nonabsorbable hydropolymer composed of 96% apyrogenic water and 4% polyalkylimide. It is currently not FDA approved, but has been available in Europe since 2001 and Health Canada approved since 2006. Two other permanent hydrogel fillers used for cosmetic facial contouring include Aquamid from Denmark and Argiform from Russia. Both are polyacrylamide polymers of different consistencies.
One must also consider that the mobility of the area in which filler is injected can have significant impact on its duration; this pertains especially to temporary fillers. In mobile areas, such as the NLF and lips, HA fillers will last up to 6 months before reinjection is needed, whereas in immobile areas such as the cheeks, the prejowl sulcus, earlobes, temples, and angle of jaw, longevity of the product approaches 12 months. According to both clinician and patient assessment, Juvéderm Voluma in the cheek area is reported to last for up to 18 months after treatment before reinjection is needed.
Moran
It depends on the location and the amount of correction performed at the time of treatment. It also depends on whether there is previous treatment to the area. Perhaps smoking plays a role. I find, also, that it seems to holds less well in patients who are already deficient in facial fat. I would guesstimate that Restylane holds 6 to 12 months, Perlane 9 to 18 months, and Juvéderm 9 to 18 months. I don’t yet have any experience with Radiesse or Sculptra. I am not certain how much permanent correction I saw when I was using Artefill. Certainly there was some, but I felt like I saw a diminution over time.
In your opinion, what are the durations of the various fillers?
Dayan
Duration of the products varies not only between the individual filler types, but also can be highly variable amongst its own brand. The HAs absorb water, gaining additional correction after being injected. HAs are reversible, metabolized via enzymatic degradation and reaction with a reactive oxygen species, and have FDA approval for 12 months on initial treatment with Juvéderm and 18 months with retreatment for Restylane. However, many experienced practitioners acknowledge incidences of correction that have extended well beyond 1 year. In fact, I have many patients in whom fullness and/or visible product is still evident at 3 years or longer. By contrast, I have others in whom it is completely resorbed within 3 months.
The reason some patients retain fillers and others rapidly metabolize it remains elusive. However, I believe a few factors beyond the individual patient’s metabolism contribute to the duration of the product. If placed into a confined nonmobile space, the product seems to last longer. I have many patients in whom I have placed a small quantity, less than 0.2 mL, into the tight space between the thick superficial musculoaponeurotic system and thin periosteum above the bony nasal dorsum, and the correction has persisted beyond 2 years. A 2007 report from the University of Michigan showed that HA can stimulate collagen production, perhaps contributing to its extended correction. In addition, I have seen many patients with worm-like lumps and bumps in the immobile NLF dermis from product placed years prior. I am certain the product is in the dermis because without any bleeding, it is easily expressed after puncturing the skin with an 18-gauge needle. It is interesting that the HA filler looks exactly like it did before being injected.
I also believe that, if placed in large quantities or if an early retreatment is given, the product seems to last longer. In 2010, we published data showing persistence of correction at 36 months with retreatment in the NLF. Although shown not to experience the fibrous ingrowth like the permanent hydrogels, it seems the HAs, with their very thin fibrous capsule, can develop a homeostatic and synergistic relationship with the surrounding tissue exchanging metabolites. Also, perhaps in greater quantities or concentration, the enlarged surface area results in inefficient metabolism of the product. I do not recognize any differences in persistence between Juvéderm and Restylane and routinely tell patients they can expect between 6 months to a year from the product, with it lasting longer in some people and shorter in others.
CaHA is metabolized by enzymatic breakdown with absorption of microspheres evident at 9 months. I find CaHA lasts predictably and routinely from 9 to 12 months, regardless of the patient. However, I did have one patient who was taking ibandronate (Boniva; Roche Therapeutics Inc, Nutley, NJ, USA) and she felt that it dissipated quicker. However, I cannot confirm this nor have I seen any scientific validation.
Poly -l -lactic acid is likely degraded by hydrolysis and extracellular enzymes, and subsequently broken down by macrophages. In my experience, it can be expected to last from 12 to 18 months following a series of 3 treatments. It is difficult, however, to gauge its persistence because the correction is so gradual and diffuse.
Artefill does provide for appreciable permanent results. However, in the NLF, where I have the most experience with the product, it seems necessary to gain complete correction first with multiple syringes, 2.0 to 3.0 mL.
Ellis
Temporary fillers are usually resorbed by the body in about 6 months to 1 year. Semipermanent fillers may last 1 to 2 years. Permanent fillers are basically nonresorbable.
Temporary fillers include:
- 1.
Bovine-derived collagen (Zyderm, Zyplast)
- 2.
Allogenic collagen (Cosmoderm, Cosmoplast, Cymetra, Fascian)
- 3.
Synthetic HA materials (Juvéderm Refine/Ultra/Ultra Plus, Restylane and Perlane, Teosyal, Esthelis, Isogel, Belotero).
Semipermanent fillers are often alloplastic in nature with the exception of Juvéderm Voluma. These fillers include:
Radiesse: an aqueous-based gel carrier with spherical particles of synthetic CaHA
Sculptra: a biodegradable poly- l- lactic acid polymer
BeautiCal: known as Outline in Europe, an absorbable polyacrylamide gel.
Juvéderm Voluma is unique in that it confers all the advantages of HA-based fillers while providing greater longevity. Juvéderm Voluma is a smooth but highly cohesive and viscous gel that uses a combination of high-MW and low-MW HA polymer chains ( Fig. 2 ). The addition of these low-MW chains improves the effectiveness of cross-linking, resulting in a product that is both cohesive and viscous ( Fig. 3 ). In vitro, this translates to a product that is able to retain its structure and resists migration.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








