Inability to achieve R0 resection [33]
Rising carcinoembryonic antigen [48]
Advanced stage of primary tumor, i.e., T4, nodal disease [40]
Recurrence-free interval <12 months [40]
High sacral involvement (above S2) [44]
Multiple points of tumor fixation within pelvis [8]
Major blood transfusion, neural invasion [33]
Cortical and marrow sacral involvement [33]
Presence of pain preoperatively- (sciatica) [40]
The contraindications for exenterative surgery for rectal and anal cancer will vary from institution to institution and from surgeon to surgeon. Table 12.2 lists what we consider to be the absolute and relative contraindications. Contraindications are based primarily on the inability to completely clear the tumor, with the understanding that limited survival benefit can be attained if gross residual tumor remains. Intraoperative radiation therapy has led to improved local control for resections that leave microscopic margins [10]. In some circumstances, the only opportunity to achieve a margin-negative resection is if extended resections that include high sacrectomy or hemipelvectomy are performed. In these circumstances, if the surgeon believes the operation can be performed safely, the patient must decide if the disability that results from surgery (i.e., lower extremity amputation and significant neuromuscular dysfunction) is an acceptable trade-off for the opportunity for cure. For these extended resections, little data exists to guide recommendations in terms of oncologic outcomes. We have approached these cases selectively and found the results encouraging [9].
Table 12.2
Absolute and relative contraindications for exenterative surgery
Absolute contraindications | Relative contraindications |
|---|---|
Unresectable distant metastasis | Extensive unilateral sidewall disease |
Bilateral pelvis side wall disease | Inability to achieve R0 resection |
Poor performance status | Sacral involvement above S3 |
Encasement of iliac vessels |
Preoperative Evaluation and Surgical Planning
When seeing patients for consideration of pelvic exenteration, it is important to start with a complete history and physical examination. Moreover, all records of previous treatments (surgical and adjuvant chemoradiation) should be reviewed. Pain and neurologic dysfunction may be signs of advanced, possibly unresectable disease [8]. Bilateral lower extremity edema is an indicator of venous or lymphatic obstruction. A full colonoscopy should be performed to rule out any synchronous colonic lesions. If the rectum is intact, a digital rectal examination can assess the relationship of the recurrent cancer to the prostate or posterior vaginal wall. In women, a gynecologic examination should be conducted to assess the extent of local invasion. Cystoscopy assesses the location and extent of bladder involvement.
Laboratory tests should be obtained looking particularly for anemia and the nutritional state of the patient. The albumin, prealbumin, and transferrin levels will provide an idea of the patient’s protein reserves and may impact postoperative morbidity and mortality. If required, nutritional supplementation should be instituted to optimize recovery, especially wound healing. In patients with colorectal cancer, a carcinoembryonic antigen (CEA) test should be performed. A rising CEA level has been associated with decreased survival after exenterative surgery (as stated earlier), and this finding should be considered in the overall decision of whether to proceed with surgery [11].
Approximately 50 % of patients presenting with locoregional recurrences of rectal cancer will have distant metastatic disease that precludes curative surgery [8]. Imaging to assess locoregional and distant disease is an essential component of the evaluation. Our protocol involves a thorough metastatic workup that includes fusion positron emission tomography (PET) and computed tomography (CT). Recent studies of 18-fluorodeoxyglucose PET imaging suggest an increased sensitivity for the detection of locoregional recurrence and metastasis from colorectal cancer [12–14]. In the evaluation of 58 patients for advanced or recurrent colorectal cancer, Ogunbiyi et al. [15] found that PET imaging had a sensitivity and specificity of 91 and 100 %, respectively. Selzner et al. [16] showed that fusion imaging that combines CT and PET imaging has an enhanced sensitivity of 89 % compared with a sensitivity of 64 % with standard CT for the detection of rectal recurrence. In their study, fusion imaging led to altered management in 21 % of patients. Moreover, PET retains its diagnostic ability even after irradiation, and we believe that all patients being considered for resection should undergo this study.
Magnetic resonance imaging (MRI) of the pelvis has become our imaging study of choice to assess neuromuscular and bony involvement and for surgical planning. MRI produces highly detailed soft-tissue resolution, which is helpful in planning lines of resection as it pertains to adjacent structures. Specifically, for tumors with posterior and lateral extension, MRI can determine proximal sacral extent, involvement of the lumbosacral nerves, and whether a margin can be obtained on the lateral pelvic side wall (Fig. 12.1). Furthermore, spinal imaging is best performed by MRI, which may demonstrate nerve root and foraminal involvement by tumor and thecal sac compression. A magnetic resonance angiogram or venogram may add additional information regarding vascular involvement and indicate the need for a vascular surgeon to be a member of the multidisciplinary surgical team.
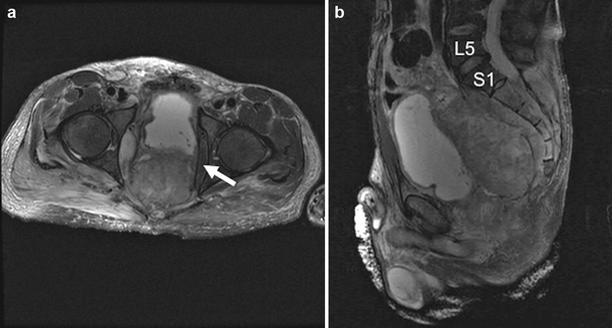

Fig. 12.1
Axial and sagittal T2-weighted magnetic resonance imaging of the pelvis in a patient with recurrent rectal cancer. Axial image demonstrates an uninvolved but very close left side wall margin (a, arrow). Sagittal image shows the proximal extent of mass involving the sacrum up to the level of the first (S1) sacral body (b)
A major challenge from a surgical planning perspective is the finding that rectal and anal cancer recurrences can be discrete and ill-defined on imaging. Recurrences may be infiltrative, sheetlike, and sometimes have islands of intervening normal tissue. This makes it difficult to preoperatively determine where the “true” clear margins lie. One must be prepared to alter the surgical plan intraoperatively because findings may differ significantly from what preoperative imaging has suggested. Because of this, we are of the opinion that frozen section pathology is necessary in cases of recurrent rectal cancer to ensure that further resection can be performed with definite negative margins. This finding is more typical for tumors with posterior and lateral extension than those with anterior extension. Surgical planning for tumors with anterior extension usually can be accomplished adequately and with good reliability with CT imaging alone (Fig. 12.2).
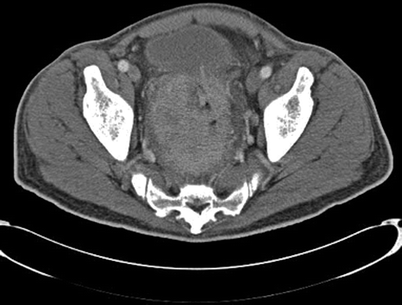

Fig. 12.2
Axial computed tomography image demonstrating a locally advanced primary rectal cancer with anterior extension to the bladder
Another challenge in the management of recurrent rectal cancer is differentiating postoperative and radiation-induced fibrosis from tumor on surveillance imaging. T2-weighted MRI can assist because fibrosis and tumor demonstrate different signal intensities [17]. Gadolinium-enhanced MRI reportedly has 87 and 100 % sensitivity and specificity, respectively, for the detection of pelvic recurrences compared with 80 % sensitivity and 86 % specificity with standard MRI [12].
There are ways to preoperatively differentiate tumor from inflammatory postoperative changes. Our approach involves comparing serial imaging, paying particular attention to changes in the size of an area of concern over time, and this is correlated with a rise in the CEA levels. CT-guided percutaneous biopsy can confirm recurrence, but a negative result does not rule out this possibility. In the absence of a tissue diagnosis, a rising CEA with a notable change in the size of the lesion on serial imaging should be considered consistent with recurrent disease.
Classification of Exenterations
The term exenteration has become ambiguous because it often is used to describe procedures that include less than a total evisceration of the pelvis. Moreover, classification schemes for exenteration do not typically include sacropelvic resection as a component. Several variations have been described, depending on the combination of organs being removed. Rodriguez-Bigas and Petrelli [18] described four types of exenteration: total pelvic, anterior, posterior, and supralevator.
For the purposes of this chapter, we have classified exenteration using the rectum or neorectum as a focal point of the malignant process. An anterior exenteration includes resection of the rectum or neorectum (if a previous low anterior resection or coloanal anastomosis was performed) and any involved viscera in front of the rectum, such as the reproductive organs, the bladder, or both (Fig. 12.3). A posterior exenteration includes the rectum or neorectum and any tissue posterior and lateral, such as musculoskeletal tissue and neurovascular structures (Fig. 12.4) An anterior-posterior exenteration involves a combination of anterior and posterolateral structures, including the bony sacropelvic components (Fig. 12.5).
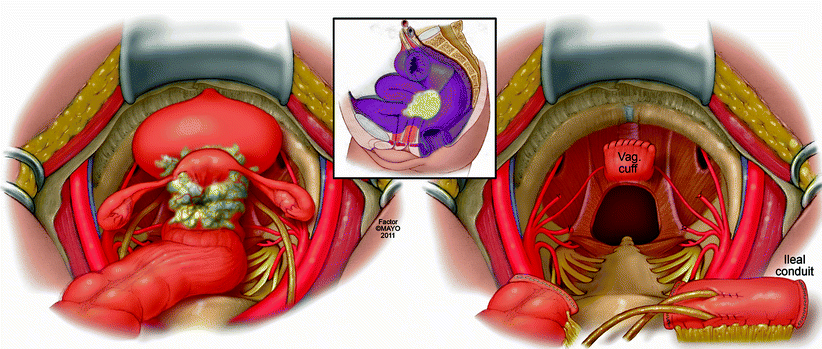
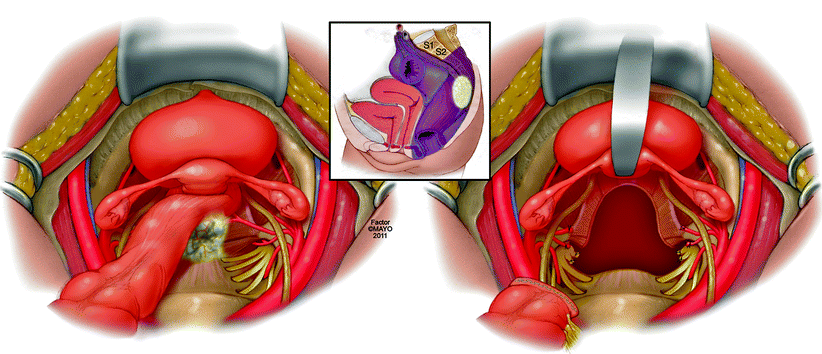
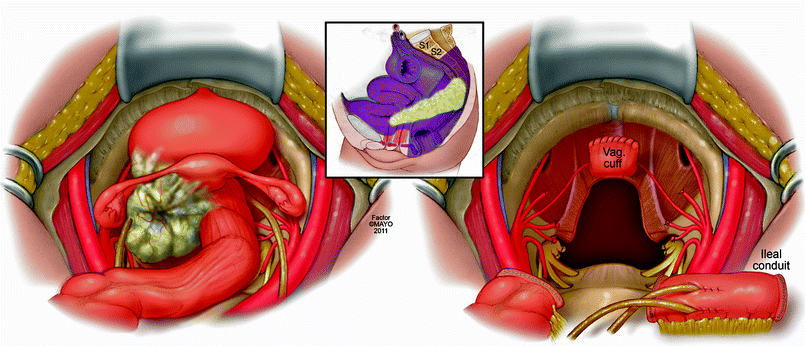

Fig. 12.3
Anterior exenteration – en bloc resection of rectum or neorectum, genitourinary, and/or gynecologic structures and postresection defect requiring reconstruction. © 2011, copyright Mayo Clinic, used with permission

Fig. 12.4
Posterior exenteration – en bloc resection of rectum or neorectum and sacrum and a postresection defect requiring reconstruction. © 2011, copyright Mayo Clinic, used with permission

Fig. 12.5
Anterior-posterior exenteration – en bloc resection of both anterior and posterior structures and postresection defect requiring reconstruction. © 2011, copyright Mayo Clinic, used with permission
Multidisciplinary Team
It is of great importance that an experienced multidisciplinary team evaluate, plan, and perform exenterative pelvic surgery. Our team consists of surgical specialists from the following disciplines: general and colorectal surgery, orthopedic oncology, orthopedic spinal surgery, urologic oncology, plastic and reconstructive surgery, and vascular surgery. Other critical team members include a musculoskeletal radiologist, a medical oncologist, a radiation oncologist trained to deliver intraoperative radiation therapy (IORT), and an experienced anesthesiologist. Safety, efficiency, and a greater opportunity for wide margin resection is facilitated by the individual expertise of the multidisciplinary surgical team members. In our opinion, a team meeting should occur before every operation to review indications, the role of adjuvant therapy, and planned lines of resection. Moreover, a debriefing meeting after extensive operations helps the team identify areas that can be improved in future cases.
Role of Multimodal Therapy
Multimodal therapy that includes systemic chemotherapy, external beam radiation, and intraoperative electron radiation therapy (IOERT) are essential components to maximize outcomes in patients with locally advanced primary or recurrent rectal cancer. Published studies have demonstrated that a 5-year survival rate between 25 and 36 % can be achieved [8, 19, 20]. Margin-free resectability may be enhanced when preoperative chemotherapy and radiation are utilized [20]. Before exenterative surgery for recurrent rectal cancer, it has been our practice to administer 50.4 Gy of external beam radiation therapy with concurrent chemotherapy if a patient has not previously received irradiation. Surgery is then planned 6–8 weeks after radiation is administered. For patients who have been previously irradiated, we deliver an additional 20–30 Gy of external beam radiation therapy before surgery [21, 22]. Preoperative chemotherapy is also administered with a 5-fluoruracil–based regimen that is modified based on the patient’s initial therapy.
Studies have demonstrated that IOERT can improve survival by improving local tumor control [10]. In our practice, the dose of IOERT given at the time of surgery depends on the resection margins. The typical IOERT dose for an R0 resection is 12.5 Gy, is 15 Gy for an R1 resection, and is 20 Gy for an R2 resection (Table 12.3). After the specimen has been resected, the surgeon, the pathologist, and the radiation oncologist evaluate the quality of the specimen and determine the areas at risk for recurrence. Our experience with IOERT has shown that when local re-recurrence takes place in the pelvis, it is most often outside the IOERT field [10].
Table 12.3
Intraoperative electron radiation therapy (IOERT) doses related to resection margin
Margin | IOERT dose (Gy) |
|---|---|
R0 (<5 mm) | 750–1,250 |
R1 | 1,000–1,500 |
Localized R2 | 1,500–2,000 |
Surgical Technique
Anterior Exenteration
We use ureteral stents for all reoperative cases and when extensive pelvic radiation has been given. The Lloyd-Davies position is used to allow access to the perineum. Care is taken to protect the extremities from nerve injury with adequate padding of both the tucked arms and lateral lower extremities in the stirrups. An exploratory laparotomy is carried out through a midline incision to confirm the absence of extrapelvic disease and to determine local resectability. Any suspicious extrapelvic lesions are biopsied and sent for frozen section analysis. Extrapelvic disease proven by biopsy may preclude pelvic resection for cure. Any adherent tissue (often from the small bowel) to the pelvic tumor should be resected en bloc.
The left colon is mobilized and transected at the appropriate level for subsequent end-colostomy. The entrance to the pelvis must be completely cleared of extraneous tissue for optimal pelvic exposure. Deep pelvic dissection to the presacral space then proceeds posteriorly along the lower aorta and continues distally over the iliac vessels and ureters (Fig. 12.6). Vasiloops are used to retract the ureters and vascular structures. In the reoperative pelvis, dense fibrosis and distorted anatomy requires careful, meticulous dissection to avoid inadvertent injury and major bleeding. The most at-risk region for significant bleeding occurs during mobilization of the left common iliac vein, and this dissection should proceed with caution. Posterior lumbar branches, if not identified, can lead to significant blood loss if avulsed.
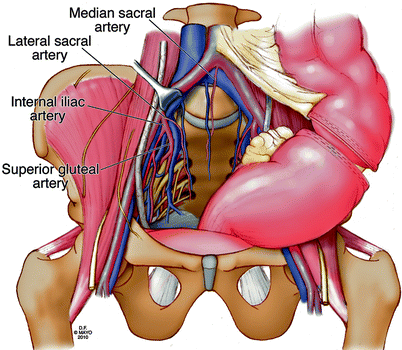

Fig. 12.6
Anatomy of presacral space after rectal mobilization. Waldeyer’s fascia is not shown to illustrate the neurovascular structures. © 2011, copyright Mayo Clinic, used with permission
The anterior and lateral lines of resection (decided upon during preoperative review of imaging) are delineated and confirmed, and the involved structures and organs are mobilized widely for subsequent en bloc resection. The presacral space is further developed, and the dissection is carried down along the anterior sacrum; the surgeon must be careful and stay anterior to Waldeyer’s fascia. The dissection is then carried laterally along the pelvic side wall on each side, with careful protection of the lumbosacral plexus and internal iliac vessels.
In women, anterior involvement may require resection of the posterior wall of the vagina, and this portion of the operation is approached transvaginally and transabdominally as a simultaneous procedure. In contrast, anterior fixation in men often requires cystoprostatectomy because of invasion of the trigone and prostate. Partial cystectomy may be sufficient in rare cases. When the bladder is resected, the ureters are mobilized as close to the bladder as possible to provide adequate length for urinary conduit reconstruction. The space of Retzius is entered to fully mobilize the anterior portion of the bladder. The blood supply to the bladder is taken by serially ligating the arteries and veins along the pelvic side wall that course back and forth from the internal iliac vessels. The wings of peritoneum to the bladder are then taken down until the bilateral vasa are identified and clipped. Then both ureters are isolated and traced down to the bladder bilaterally and are subsequently clipped and brought out of the pelvis. The distal margins are sent and confirmed to be negative by pathology. With the ureters both isolated and out of the pelvis, the pedicles are taken down to the bladder and prostate. The focus then is on the anterior dissection. The endopelvic fascia is opened bilaterally. The dorsal venous complex is subsequently ligated. The urethra is reached, and the catheter is delivered into the wound and the urethra is transected.
Creation of an end-colostomy and a urinary conduit completes the procedure. In women who require posterior vaginal wall removal, reconstruction may be necessary. For anterior resection, soft-tissue reconstruction to fill a void in the pelvis is used selectively.
Posterior Exenteration (Sacrectomy)
First Stage: Anterior Approach
After placement of ureteral stents, an exploratory laparotomy is carried out through a midline incision to confirm the absence of extrapelvic disease and to determine local resectability. The left colon is mobilized and transected at the appropriate level to be used later for colostomy, exposing the presacral space. Deep pelvic dissection then proceeds posteriorly along the lower aorta and continues distally over the iliac vessels and ureters. The anterior and lateral lines of resection (decided upon during preoperative review of imaging) are delineated, and the involved structures and organs are mobilized widely for subsequent en bloc resection. Frozen section biopsies are taken as needed to establish that margins are negative at the level of the proximal sacral transection.
Vascular exposure often requires mobilization of the lower aorta and vena cava, in addition to the iliac arteries and veins. Circumferential mobilization of the common and external iliac arteries may be needed to facilitate exposure of the veins. The internal iliac artery branches are ligated and divided first. This is performed distal to the takeoff of the posterior division of the superior gluteal artery branch to preserve blood flow to the gluteal flaps (Fig. 12.7). Multiple internal iliac vein branches are ligated after control of the main trunk(s) of the internal iliac vein has been achieved. The branches are ligated and divided before ligation of the main trunk to avoid venous distension, which can lead to troublesome bleeding. Lateral and middle sacral vein branches, which drain into the posterior aspect of the left common iliac vein and caval confluence, are ligated and divided. Suture ligature is preferable for short, broad-based internal iliac vein branches. The vascular dissection is carried along both sides of the sacrum onto the pelvic floor.
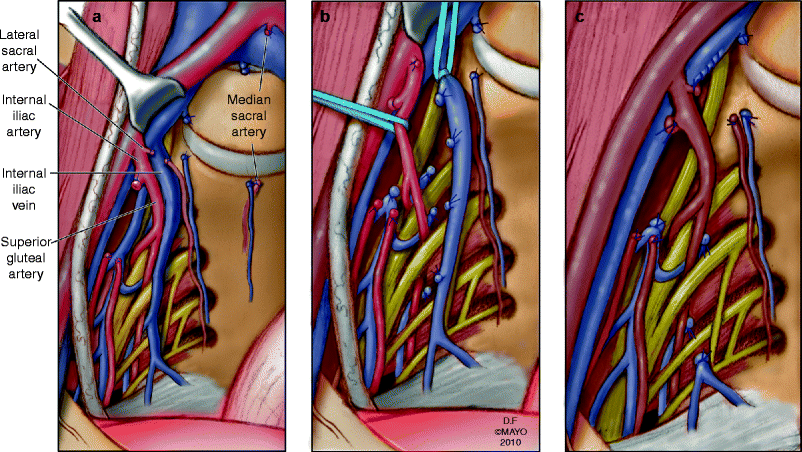

Fig. 12.7
Vascular exposure and dissection. Internal iliac artery branches are ligated, preserving gluteal blood flow (a). Ligation of the lateral sacral branches of the internal iliac vein, all the way down to the distal sacrum (b). High ligation of the internal iliac vein (c). © 2010, copyright Mayo Clinic, used with permission
During this anterior stage of the operation, a colostomy and an ileal or colonic urinary conduit are fashioned as needed. A myocutaneous flap from the rectus abdominis is then prepared for subsequent perineal reconstruction. Using intraoperative fluoroscopy, the sacral level of transection is determined and anterior osteotomies are performed. A screw is placed in the osteotomy site to facilitate alignment for fluoroscopy (Fig. 12.8). Before closing and turning the patient, a thick silastic mesh is placed anterior to the sacrum and posterior to the vessels and soft tissue structures to protect against injury when blind osteotomies are performed during the second stage (prone) of the procedure (Fig. 12.9). The abdomen is then closed, stomas are matured (fecal, urinary), and the patient is turned and placed in the prone position.
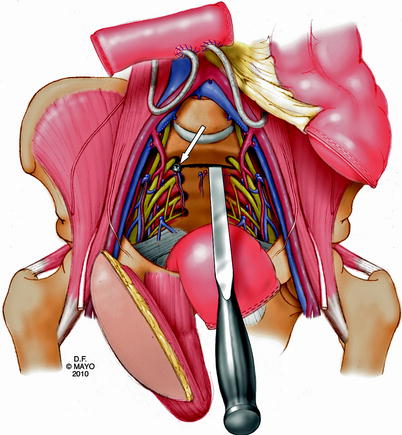
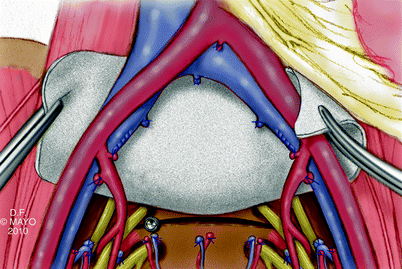

Fig. 12.8
Unicortical transverse osteotomy, marked with a “screw” (arrow) to facilitate alignment with fluoroscopy during posterior dissection. © 2010, copyright Mayo Clinic, used with permission

Fig. 12.9
Placement of silastic mesh to protect anterior vasculature when posterior osteotomies are performed blindly. © 2010, copyright Mayo Clinic, used with permission
Second Stage: Posterior Approach
The second stage of the procedure is carried out with the patient in the prone position. A posterior midline incision is made along the mid-portion of the sacrum, and the gluteus maximus muscles are dissected away from the sacral attachments. The sacrospinous and sacrotuberous ligaments are divided to access the pelvic cavity posteriorly (Fig. 12.10). The piriformis muscle is divided while protecting the sciatic and pudendal nerves. Laminectomy, dural sac ligation, and sacral resection are then carried out (Fig. 12.11). Final osteotomies are performed based on imaging studies that assure tumor-free margins. After resection, the specimen is examined by the pathologist and the surgical team to assess the completeness of resection. The posterior wound is then reconstructed with a pedicled myocutaneous rectus flap (Fig. 12.12




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








